1695
MRF for preoperative differentiation of non-functioning gonadotroph adenoma and non-functioning non-gonadotropin adenoma1Henan provincial people's hospital& Zhengzhou University People’s Hospital, Zhengzhou, China, 2Henan provincial people's hospital, Zhengzhou, China, 3MR Pre-development, Siemens Healthcare, Erlangen, Germany, 4MR Collaboration, Siemens Healthcare Ltd, Beijing, China
Synopsis
Currently, there is no effective pharmacological treatment for non-functioning pituitary adenoma, including gonadotroph adenoma (GPA). Recent findings showed that an alternative pharmacological treatment targeted at somatostatin receptor 3 may be promising, which required accurate diagnosis of GPA before surgery. This study aimed to evaluate the utility of magnetic resonance fingerprinting (MRF) in the pre-surgical differentiation of GPA from non-functioning, non-gonadotropin adenoma (NGPA). The results showed that GPAs have significantly higher T1 and T2 values in the solid tumor than NGPA, suggesting that MRF may have potential for diagnosing GPA and benefit its treatment plan.
Introduction
Currently, there is no effective pharmacological treatment for non-functioning pituitary adenoma (NFPA), including gonadotroph pituitary adenoma (GPA), which is the most prevalent subtype of NPFA. The therapeutic alternatives include surgery and post-operative radiation. Recent studies found a high expression of somatostatin receptor 3 (SSTR3) in GPA and suggested that pasireotide with SSTR3 as the target is a promising alterative pharmacological treatment [1], thus avoiding unnecessary surgical damage. This pharmacological treatment requires the accurate differential diagnosis of GPA before surgery. However, the diagnosis of GPA relies on histological characterization. Conventional imaging cannot differentiate GPA from non-gonadotropin pituitary adenoma (NGPA) before surgery, which may prohibit the application of this treatment. Magnetic resonance fingerprinting (MRF) is a novel technique that enables the direct derivation of tissue-characteristic quantities such as proton density and relaxation constants T1 and T2. Therefore, this study aimed to evaluate the utility of MRF in the preoperative differentiation of GPA and NGPA.Methods
Twenty-three subjects (10 males; mean age: 55.1 years) with pathologically confirmed, non-functioning adenomas were enrolled. All the subjects were scanned before surgery on a 3T MAGNETOM Skyra (Siemens Healthcare, Erlangen, Germany) scanner using a 20-channel head/neck coil. The MR protocol included the following sequences: conventional MRI (2D T1-weighted, 2D T2-weighted) and a prototype spiral fast imaging with steady-state precession (FISP) MRF (FOV = 256 x 256 mm2; matrix = 256 x 256; slice thickness = 5 mm; flip angle variable = 0° - 74°; random TR between 12.1 ms and 15.0 ms; 3000 measurements; and 41 s/slice; 18 slices). The quantitative T1 and T2 maps were generated by matching the measured MRF signal time course to the dictionary. According to immunohistochemistry results, the patients were divided into a GPA group (n = 10) and NGPA (n = 13) group. Two radiologists blinded to the clinical information analyzed the data independently. Regions of interests (ROIs) were manually drawn on the tumor. The Mann-Whitney U test was used to compare the T1 and T2 values in the solid tumor between the GPA group and NGPA group. Significance was set at P < 0.05. Receiver operating characteristic (ROC) curves were devised to evaluate the diagnostic performance.Results
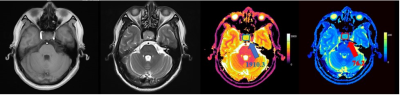
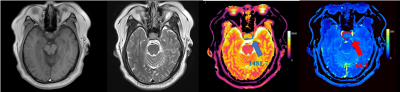
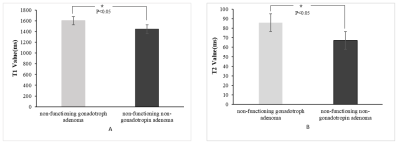
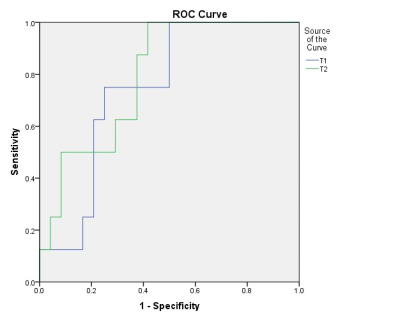
MR images and T1 and T2 values for one patient with GPA and another patient with NGPA are shown in Figure 1 and Figure 2, respectively. The conventional MR images showed little difference, whereas the quantitative T1 and T2 maps could provide more information for a differential diagnosis. As shown in Figure 3, the GPAs had significantly higher T1 values (mean ± standard deviation, 1603[BM(DMM1] ± 163 vs. 1445 ± 202 ms, P = 0.013) and T2 value (mean ± standard deviation, 86 ± 21 vs. 67 ± 19 ms, P = 0.041) than NGPA. The ROC curves for each modality are shown in Figure 4. The area under the ROC curves of T1 and T2 were 0.745 and 0.792, respectively. [BM(DMM1]Avoid pseudo-accuracy that is not justified.Discussion
As the most prevalent subtype of NPFA, GPA often presents as invasive macro-adenoma not amenable to complete surgical resection. Radiotherapy is the only post-operative option for patients with large invasive or recurrent lesions. Recent findings suggest that SSTR3-targeted pharmacological treatment is a promising alternative to standard primary surgery for GPA [1, 2]. Therefore, a method that could accurately differentiate GPA from NGPA before surgery is essential and will benefit the treatment plan for GPA. Our results showed that GPA had significantly higher T1 and T2 values in the solid tumor than NGPA. Moreover, both the T1 and T2 values showed good diagnostic performance, with T2 demonstrating slightly better diagnostic efficacy, indicating that MRF as a completely non-invasive and quantitative imaging method may have the potential for diagnosing gonadotroph adenoma before surgery, which will benefit the treatment plan and thus avoid unnecessary surgery damage with this lesion.Conclusion
MRF provides a non-invasive imaging method for the preoperative differentiation of non-functioning gonadotroph adenoma and non-functioning non-gonadotropin adenoma. The quantitative information yield by MRF may have a potential for guiding the treatment plan of non-functioning gonadotroph adenoma in the future.Acknowledgements
This research was supported by the National Key R&D Program of China (2017YFE0103600), National Natural Science Foundation of China (81720108021, 81601466), and Zhongyuan Thousand Talents Plan Project-- Basic Research Leader Talent (ZYQR201810117).References
1. Lee M, Lupp A, Mendoza N, Martin N, Beschorner R, Honegger J, Schlegel J, Shively T, Pulz E, Schulz S et al: SSTR3 is a putative target for the medical treatment of gonadotroph adenomas of the pituitary. Endocr Relat Cancer 2015, 22(1):111-119.
2. Taboada GF, Luque RM, Bastos W, Guimaraes RF, Marcondes JB, Chimelli LM, Fontes R, Mata PJ, Filho PN, Carvalho DP et al: Quantitative analysis of somatostatin receptor subtype (SSTR1-5) gene expression levels in somatotropinomas and non-functioning pituitary adenomas. Eur J Endocrinol 2007, 156(1):65-74.
Figures