1688
Diffusion and multi-echo ASL reveal lower blood-brain interface water permeability in mild cognitive impairment and early Alzheimer’s disease1Auckland Bioengineering Institute, University of Auckland, Auckland, New Zealand, 2Brain Research New Zealand, Auckland, New Zealand, 3Stevens Neuroimaging and Informatics Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 4Fraunhofer Institute for Digital Medicine MEVIS, Bremen, Germany, 5Faculty 01 (Physics/Electrical Engineering), University Bremen, Bremen, Germany, 6School of Psychology, University of Auckland, Auckland, New Zealand, 7Centre for Brain Research, University of Auckland, Auckland, New Zealand, 8Department of Pharmacology, University of Auckland, Auckland, New Zealand, 9Dementia Research Centre, UCL Queen Square Institute of Neurology, University College London, London, United Kingdom, 10Wellcome Centre for Human Neuroimaging, UCL Queen Square Institute of Neurology, University College London, UK, London, United Kingdom, 11Department of Engineering Science, University of Auckland, Auckland, New Zealand
Synopsis
Blood-brain barrier (BBB) dysfunction has been found in multiple neurodegenerative conditions, including mild cognitive impairment (MCI) and Alzheimer’s disease (AD). However recent concerns on the repeated use of Gadolinium based contrast agents (GBCAs), prompted us to investigate alternative, non-invasive methods for measuring BBB function. Both diffusion-weighted (DW) and multi-echo (ME) ASL was implemented at 3T to determine water transfer rates (kw) in the brain in MCI and early AD participants. We found kw to be lower in the cognitively impaired group, compared to controls, with both modalities, suggesting that these techniques may provide a marker of early AD pathology.
INTRODUCTION
Recent studies using dynamic contrast-enhanced (DCE) MRI have reported subtle increases in the permeability of the blood-brain barrier (BBB) to gadolinium based contrast agents in mild cognitive impairment (MCI) and Alzheimer’s disease (AD) [1,2]. BBB breakdown in AD has also been confirmed in post-mortem tissue, which supports the ‘leaky’ BBB findings posited by the DCE-MRI studies [3,4].The past decade has seen growing interest in the use of Arterial Spin Labelling (ASL) MRI as a non-invasive alternative to DCE-MRI to detect changes in barrier permeability (reviewed in [5]). Because of the small size of water molecules relative to gadolinium chelates, ASL may have greater sensitivity to changes in paracellular ‘leakiness’ of the BBB. However, water is also transported via transcellular routes across the greater neurovascular unit, or blood-brain interface (BBI). Water transfer across the BBI, as measured with ASL, may therefore capture different changes in BBI function than DCE-MRI.
Diffusion-weighted (DW) ASL [7,8] and multi-echo (ME) ASL [9-11] are two alternative methods that have been proposed to investigate BBI permeability. In this work we implemented and applied DW-ASL and ME-ASL to investigate the water transfer rates in grey-matter in MCI/AD and age-matched participants.
METHODS
All images were acquired using a MAGNETOM Skyra 3T MR scanner (Siemens Healthcare, Erlangen, Germany) with a 32-channel head coil. DW-ASL imaging was performed as previously described [7], namely pCASL labelling with two post-labelling delays (PLD) of 900 and 1800 ms, and multiple repeats at three different diffusion weightings (b-values: 0, 14, 50 s/mm2), yielding a total scan time of approximately 7’30’’. A pCASL labelling scheme was also used for ME-ASL, which included a multi-PLD scan for bolus arrival time (BAT) estimation (bolus duration: 400 ms, 7xPLDs: 500-2500ms, 400ms increments TE: 20.54ms) and a Hadarmard-encoded multi-echo, multi-PLD perfusion scan (3xPLDs: 1100, 2100, 3100ms, 7xTEs: 21-271ms, 42ms increments), with 2 repeats, also yielding a scan time of approximately 7’30’’.Twenty one participants enrolled in the Dementia Prevention Research Clinic (Auckland, New Zealand) were recruited. All participants were clinically diagnosed following a multi-disciplinary assessment including the Addenbrooke's Cognitive Examination-III (ACE-III), neuropsychological testing and neuro-imaging. Classifications included single- and multi-domain MCI and early AD. For statistical analysis, these participants were combined into a “cognitively imparied” (CI) group. Five participants were excluded from the DW-ASL analysis due to low labelling efficiency caused by incorrect positioning of the labelling plane. Total grey-matter arterial transit time (ATT), cerebral blood flow (CBF) and water transfer rate (kw) were determined from the DW-ASL data for each of the remaining CI participants (n=9) and cognitively normal (CN) participants (n=7) using previously published methods [7]. Two participants were excluded from the ME-ASL analysis as the fitting algorithms failed to converge, and one was excluded because of missing data. For the remaining CI (n=13) and CN (n=5) participants, OxfordASL (v3.9.17) was used to fit a single-compartment model to the BAT scans to determine ATT and CBF [12]. A previously published two-compartment exchange model developed for pASL [10] was modified for pCASL in this work. Exchange model equations for the ME-ASL signal were solved using MATLAB’s Symbolic Maths Toolbox (v8.1) and fitted to the grey-matter masked multi-PLD multi-echo data in a least-squares sense to estimate kw.
RESULTS
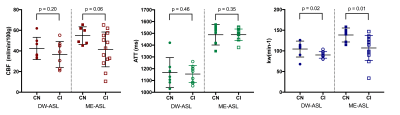
Statistically significant differences in kw between the CI and CN groups were found using both DW-ASL and ME-ASL scans (Figure 1). No significant between-group differences were found for ATT or CBF, although the CI group appears to have a slightly lower mean CBF for both imaging modalities.In the CN group, ME-ASL estimates of kw (136±8/min (mean±SE) ) and CBF (57±4 ml/100g/min) were slightly higher than DW estimates (kw:104±7/min, CBF:42±4 ml/100g/min). In the CI group, ME kw and CBF estimates (kw:107±8/min, CBF:42±5 ml/100g/min) were in a similar range to DW-ASL estimates (kw:90±3/min, CBF: 36±4 ml/100g/min). Fitted values for ATT differed between the ME-ASL (ATT: 1490±10 ms CI, 1500±40 ms CN) and DW-ASL (ATT: 1150±20 ms CI, 1170±50 ms CN) modalities. This is most likely caused by methodological differences in how ATT is estimated between the two modalities.
Figure 2 shows positive correlations between CBF and cognitive score, and kw and cognitive score for both imaging modalities. There was no correlation between ATT and ACE score with either method.
DISCUSSION AND CONCLUSIONS
In this work we compared for the first time, DW-ASL and ME-ASL to measure water transfer rates in cognitively impaired and age-matched cognitively normal participants. Values for water transfer rate, kw, using both methods were similar, providing reassurance of the accuracy of the measurements. While CBF and ATT were not significantly different between groups, we found a reduction in kw in CI compared to CN participants. This result is contrary to what would be expected according to the ‘leaky’ barrier findings of DCE-MRI studies. Our pilot results suggest an overall decrease in water transfer rates across the BBI in CI participants, which may reflect a decrease in transcellular water transport due to changes in astrocyte AQP4 distribution. Indeed, a reduction in water transfer rates, measured using similar methods to those applied here, have been found in a AQP4 knock-out model of AD [11]. Recruitment for this study is ongoing, and increasing the size of the cohort will enable further validation of the results presented here.Acknowledgements
This work is funded by Brain Research New Zealand.TJM is supported by a University of Auckland Doctoral Scholarship and a Brain Research New Zealand travel grant.DLT is supported by the Wolfson Foundation (PR/ylr/18575), NIHR UCL/H Biomedical Research Centre, and Wellcome Trust.We would like to thank Jane Govender from the Dementia Prevention Research Clinic for all her help with recruitment, and the MR technologists at the Centre for Advanced MRI (CAMRI) for participant scanning.References
[1] Van de Haar, Harm J., Saartje Burgmans, Jacobus F. A. Jansen, Matthias J. P. van Osch, Mark A. van Buchem, Majon Muller, Paul A.M. Hofman, Frans R.J. Verhey, and Walter H. Backes. “Blood-Brain Barrier Leakage in Patients with Early Alzheimer Disease.” Radiology 281, no. 2 (May 31, 2016): 527–35. https://doi.org/10.1148/radiol.2016152244.
[2] Montagne, Axel, Samuel R. Barnes, Melanie D. Sweeney, Matthew R. Halliday, Abhay P. Sagare, Zhen Zhao, Arthur W. Toga, et al. “Blood-Brain Barrier Breakdown in the Aging Human Hippocampus.” Neuron 85, no. 2 (January 21, 2015): 296–302. https://doi.org/10.1016/j.neuron.2014.12.032.
[3] Narayan, Pritika J., Sue-Ling Kim, Claire Lill, Sheryl Feng, Richard L. M. Faull, Maurice A. Curtis, and Michael Dragunow. “Assessing Fibrinogen Extravasation into Alzheimer’s Disease Brain Using High-Content Screening of Brain Tissue Microarrays.” Journal of Neuroscience Methods 247 (May 30, 2015): 41–49. https://doi.org/10.1016/j.jneumeth.2015.03.017.
[4] Sweeney, Melanie D., Abhay P. Sagare, and Berislav V. Zlokovic. “Blood–Brain Barrier Breakdown in Alzheimer’s Disease and Other Neurodegenerative Disorders.” Nature Reviews. Neurology 14, no. 3 (March 2018): 133–50. https://doi.org/10.1038/nrneurol.2017.188.
[5] Dickie, Ben R., Geoff J. M. Parker, and Laura M. Parkes. “Measuring Water Exchange across the Blood-Brain Barrier Using MRI.” Progress in Nuclear Magnetic Resonance Spectroscopy, September 12, 2019. https://doi.org/10.1016/j.pnmrs.2019.09.002.
[6] Zeppenfeld DM, Simon M, Haswell JD, et al. Association of Perivascular Localization of Aquaporin-4 With Cognition and Alzheimer Disease in Aging Brains . JAMA Neurol. 2017;74(1):91–99. doi:10.1001/jamaneurol.2016.4370
[7] Shao, Xingfeng, Samantha J. Ma, Marlene Casey, Lina D’Orazio, John M. Ringman, and Danny J. J. Wang. “Mapping Water Exchange across the Blood–Brain Barrier Using 3D Diffusion-Prepared Arterial Spin Labeled Perfusion MRI.” Magnetic Resonance in Medicine 81, no. 5 (2019): 3065–79. https://doi.org/10.1002/mrm.27632.
[8] St. Lawrence, K., D. Owen, and D.J.J. Wang. “A Two-Stage Approach for Measuring Vascular Water Exchange and Arterial Transit Time by Diffusion-Weighted Perfusion MRI.” Magnetic Resonance in Medicine 67, no. 5 (2012): 1275–84. https://doi.org/10.1002/mrm.23104.
[9] Wells, Jack A, Bernard Siow, Mark F Lythgoe, and David L Thomas. “Measuring Biexponential Transverse Relaxation of the ASL Signal at 9.4 T to Estimate Arterial Oxygen Saturation and the Time of Exchange of Labeled Blood Water into Cortical Brain Tissue.” Journal of Cerebral Blood Flow & Metabolism 33, no. 2 (February 2013): 215–24. https://doi.org/10.1038/jcbfm.2012.156.
[10] Gregori, Johannes, Norbert Schuff, Rolf Kern, and Matthias Günther. “T2-Based Arterial Spin Labeling Measurements of Blood to Tissue Water Transfer in Human Brain.” Journal of Magnetic Resonance Imaging 37, no. 2 (February 1, 2013): 332–42. https://doi.org/10.1002/jmri.23822.
[11] Ohene, Yolanda, Ian F. Harrison, Payam Nahavandi, Ozama Ismail, Eleanor V. Bird, Ole P. Ottersen, Erlend A. Nagelhus, David L. Thomas, Mark F. Lythgoe, and Jack A. Wells. “Non-Invasive MRI of Brain Clearance Pathways Using Multiple Echo Time Arterial Spin Labelling: An Aquaporin-4 Study.” NeuroImage 188 (March 1, 2019): 515–23. https://doi.org/10.1016/j.neuroimage.2018.12.026.
[12] Chappell, M. A., A. R. Groves, B. Whitcher, and M. W. Woolrich. “Variational Bayesian Inference for a Nonlinear Forward Model.” IEEE TRANSACTIONS ON SIGNAL PROCESSING 57, no. 1 (2009). https://ora.ox.ac.uk/objects/uuid:1e31142d-b892-4910-807f-7cf44f290a9b.
Figures