1686
Enlarged Perivascular Spaces in Older Adults: Segmentation by Deep Learning; Correlations with Neuropathology and Cognitive Decline1Biomedical Engineering, Illinois Institute of Technology, Chicago, IL, United States, 2Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago, IL, United States, 3Computer Science, Illinois Institute of Technology, Chicago, IL, United States
Synopsis
Enlarged perivascular spaces (EPVS) are common in aging and have been linked to increased risk of stroke, lower cognitive function, and vascular dementia. However, the neuropathologic correlates of EPVS, as well as the contributions of EPVS to cognition, are not well understood. In this work, we first developed a deep-learning algorithm for automatic segmentation and full quantification of whole brain and regional EPVS, and then studied the neuropathologic correlates of EPVS as well as the contributions of EPVS on cognition in a large community-based cohort of older adults.
INTRODUCTION
Enlarged perivascular spaces (EPVS) are common in aging and have been linked to increased risk of stroke1–6, lower cognitive function7–9, and vascular dementia9,10. However, the neuropathologic correlates of EPVS, as well as the contributions of EPVS to cognition, are not well understood11. The first goal of this study was to develop a deep-learning algorithm for automatic segmentation of EPVS, as no automated segmentation approach is publicly available. The new segmentation method allowed full quantification of EPVS in the whole brain and regionally. The second goal of this work was to investigate the neuropathologic correlates of EPVS (whole brain and regional) as well as the contributions of EPVS to cognition in a large community-based cohort of older adults.METHODS
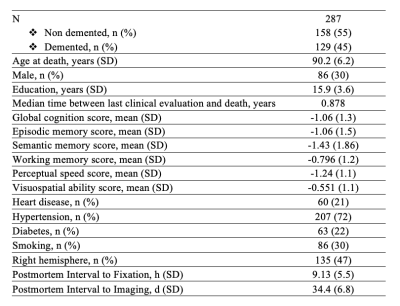
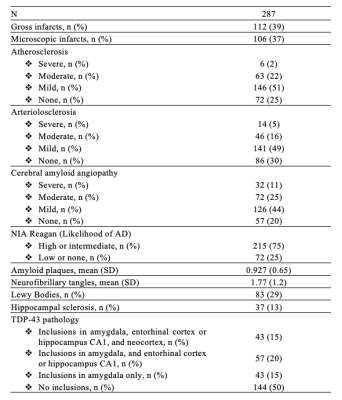
Participants and dataThis work included 287 participants of the Rush Memory and Aging Project12 and Religious Orders Study13, two longitudinal cohort studies of aging (Fig.1). All participants underwent annual clinical evaluation and cognitive assessment, ex-vivo MRI, and neuropathologic examination. Ex-vivo T2-weighted images were collected for one brain hemisphere from each participant, while the hemisphere was immersed in 4% formaldehyde solution, using a clinical 3T MRI scanner and a spin-echo sequence with 0.6x0.6x1.5 mm3 voxel-size. The average postmortem interval to fixation was PMIf≈9 hours, and the average postmortem interval to imaging PMIi≈39 days. Following ex-vivo MRI, all hemispheres underwent detailed neuropathologic examination by a board-certified neuropathologist blinded to clinical and imaging findings (Fig.2). The pathologies assessed included: gross and microscopic infarcts, atherosclerosis, arteriolosclerosis, cerebral amyloid angiopathy (CAA), amyloid plaques, neurofibrillary tangles, hippocampal sclerosis, Lewy bodies, and TDP-43.
Segmentation by deep learning
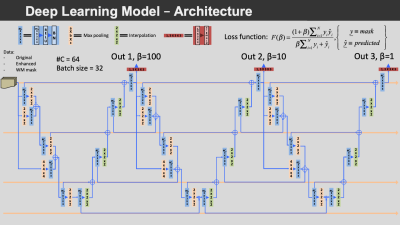
The data from 10 participants with varying EPVS severity and manually segmented EPVS were used for training convolutional neural networks (CNNs) to automatically segment EPVS. The proposed CNNs included three concatenated M2EDN14 U-Nets (Fig.3). The trained networks were used to segment EPVS in the ex-vivo MRI data of all participants.
EPVS quantification
The total volume of EPVS normalized by the total white matter volume (EPVSvsWM) was calculated for each participant. The total number of EPVS (EPVSnum) was also quantified. All brain hemispheres were also divided into lobes excluding the basal ganglia which was a separate subregion, and similar measurements were made per lobe and basal ganglia (e.g. EPVSvsWM-parietal: EPVS volume in the parietal lobe normalized by the white matter volume in the parietal lobe; and EPVSnum-parietal: number of EPVS in the parietal lobe). All EPVS measures were square root transformed to reduce skewness.
Statistical analysis
Linear regression was used to investigate associations of EPVS volume and number (dependent variables), both whole brain and regional, with neuropathologies (independent variables) in a two-step approach. First, single pathology models were tested, and pathologies having p-value<0.1 (marginally significant) were identified. Next, all pathologies identified in the previous models were included in the same multiple linear regression model controlling for demographics, PMIf and PMIi. Linear mixed-effects models controlling for all pathologies and demographics were used to investigate the independent association of cognition and cognitive decline with EPVS, above and beyond what was explained by neuropathologies and demographics. The same analysis was repeated for global cognition and five cognitive domains: episodic memory, semantic memory, working memory, perceptual speed, and visuospatial abilities.
RESULTS
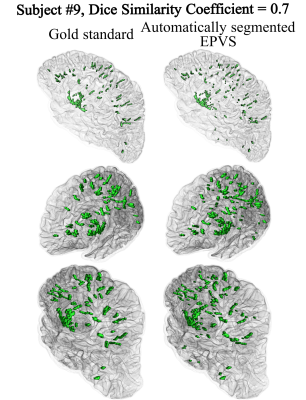
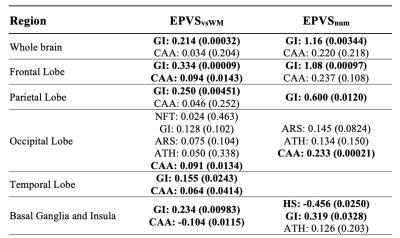
The mean Dice Similarity Coefficient on validation subjects during leave one out cross validation (LOOCV) was 0.655±0.058, and a comparison of manual and automatic segmentation can be seen in Figure 4. Whole brain normalized EPVS volume and number was associated with gross infarcts (Fig.5). Regional EPVS were associated with gross infarcts in most of the brain, and with cerebral amyloid angiopathy mainly in the occipital and temporal lobes (Fig.5). Normalized EPVS volume in the occipital lobe was negatively correlated with semantic memory (-0.443, p=0.03) and perceptual speed (-0.311, p=0.048) above and beyond what was explained by pathologies and demographics.DISCUSSION
The present work is the first to combine full quantification of EPVS, detailed neuropathologic examination, and longitudinal cognitive assessment in a large community-based cohort of older adults. This study generated robust evidence that EPVS are associated with gross infarcts and cerebral amyloid angiopathy. Although the exact mechanisms behind the observed associations are not fully understood, EPVS and the two pathologies may share similar neurobiological pathways. For example, the various etiologies that have been proposed for EPVS may also precipitate ischemia and infarction11,15. Also, amyloid deposition in cortical vessels may impair interstitial fluid drainage, causing retrograde dilation of perivascular spaces in underlying white matter16,17. Additionally, the present work demonstrated independent contributions of EPVS on cognition above and beyond the contributions of neuropathologies and demographics, suggesting that EPVS capture additional tissue damage not explained by neuropathologies.CONCLUSION
This investigation provides robust evidence on the neuropathologic correlates of EPVS and the contributions of EPVS on cognition in a large community-based cohort of older adults. Fully quantitative assessment of EPVS was facilitated by EPVS segmentation using deep learning. EPVS were shown to have associations with gross infarcts and cerebral amyloid angiopathy, and independent contributions on cognition above and beyond those of neuropathologies and demographics.Acknowledgements
This study was supported by National Institutes of Health grants P30AG010161, UH2NS100599, UH3NS100599, R01AG064233, RF1AG022018, R01AG056405, R01AG042210, R01AG17917.
The authors would like to thank the participants and staff of the Rush University Memory and Aging Project, and Religious Orders Study.
References
- Rouhl RPW, van Oostenbrugge RJ, Knottnerus ILH, Staals JEA, Lodder J. Virchow-Robin spaces relate to cerebral small vessel disease severity. J Neurol. 2008;255(5):692-696. doi:10.1007/s00415-008-0777-y
- Zhu Y-C, Tzourio C, Soumare A, Mazoyer B, Dufouil C, Chabriat H. Severity of Dilated Virchow-Robin Spaces Is Associated With Age, Blood Pressure, and MRI Markers of Small Vessel Disease: A Population-Based Study. Stroke. 2010;41(11):2483-2490. doi:10.1161/STROKEAHA.110.591586
- Hurford R, Charidimou A, Fox Z, Cipolotti L, Jager R, Werring DJ. MRI-visible perivascular spaces: relationship to cognition and small vessel disease MRI markers in ischaemic stroke and TIA. J Neurol Neurosurg Psychiatry. 2014;85(5):522-525. doi:10.1136/jnnp-2013-305815
- Doubal FN, MacLullich AMJ, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged Perivascular Spaces on MRI Are a Feature of Cerebral Small Vessel Disease. Stroke. 2010;41(3):450-454. doi:10.1161/STROKEAHA.109.564914
- Potter GM, Doubal FN, Jackson CA, et al. Enlarged Perivascular Spaces and Cerebral Small Vessel Disease. Int J Stroke. 2015;10(3):376-381. doi:10.1111/ijs.12054
- Selvarajah J, Scott M, Stivaros S, et al. Potential surrogate markers of cerebral microvascular angiopathy in asymptomatic subjects at risk of stroke. Eur Radiol. 2009;19(4):1011-1018. doi:10.1007/s00330-008-1202-8
- MacLullich AMJ. Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J Neurol Neurosurg Psychiatry. 2004;75(11):1519-1523. doi:10.1136/jnnp.2003.030858
- Huijts M, Duits A, Staals J, Kroon A, Leeuw P, Oostenbrugge R. Basal Ganglia Enlarged Perivascular Spaces are Linked to Cognitive Function in Patients with Cerebral Small Vessel Disease. Curr Neurovasc Res. 2014;11(2):136-141. doi:10.2174/1567202611666140310102248
- Uggetti C, Egitto MG, Pichiecchio A, et al. Subcortical Dementia Associated with Striking Enlargement of the Virchow-Robin Spaces and Transneural Degeneration of the Left Mammillo-Thalamic Tract. Cerebrovasc Dis. 2001;12(4):287-290. doi:10.1159/000047722
- Patankar TF, Mitra D, Varma A, Snowden J, Neary D, Jackson A. Dilatation of the Virchow-Robin Space Is a Sensitive Indicator of Cerebral Microvascular Disease: Study in Elderly Patients with Dementia. 2005:9.
- Brown R, Benveniste H, Black SE, et al. Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc Res. 2018;114(11):1462-1473. doi:10.1093/cvr/cvy113
- A. Bennett D, A. Schneider J, S. Buchman A, L. Barnes L, A. Boyle P, S. Wilson R. Overview and Findings from the Rush Memory and Aging Project. Curr Alzheimer Res. 2012;9(6):646-663. doi:10.2174/156720512801322663
- A. Bennett D, A. Schneider J, Arvanitakis Z, S. Wilson R. Overview and Findings from the Religious Orders Study. Curr Alzheimer Res. 2012;9(6):628-645. doi:10.2174/156720512801322573
- Lian C, Zhang J, Liu M, et al. Multi-channel multi-scale fully convolutional network for 3D perivascular spaces segmentation in 7T MR images. Med Image Anal. 2018;46:106-117. doi:10.1016/j.media.2018.02.009
- Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12(5):483-497. doi:10.1016/S1474-4422(13)70060-7
- van Veluw SJ, Biessels GJ, Bouvy WH, et al. Cerebral amyloid angiopathy severity is linked to dilation of juxtacortical perivascular spaces. J Cereb Blood Flow Metab. 2016;36(3):576-580. doi:10.1177/0271678X15620434
- Charidimou A, Jaunmuktane Z, Baron J-C, et al. White matter perivascular spaces: An MRI marker in pathology-proven cerebral amyloid angiopathy? Neurology. 2014;82(1):57-62. doi:10.1212/01.wnl.0000438225.02729.04
Figures