1685
Association of cerebral microbleeds and blood-brain barrier function in patients with different severity levels of dementia1Institute of Brain Science, School of Medicine, National Yang-Ming University, Taipei, Taiwan, 2Institute of Information Science, Academia Sinica, Taipei, Taiwan, 3Department of Radiology, Taipei Veterans General Hospital, Taipei, Taiwan, 4Department of Neurology, Taipei Veterans General Hospital, Taipei, Taiwan, 5Department of Neurology, National Yang Ming University, Taipei, Taiwan, 6Department of Neurology, Neurological Institute, Taipei Veterans General Hospital, Taipei, Taiwan, 7Faculty of Medicine, National Yang-Ming University School of Medicine, Taipei, Taiwan, 8Brain Research Center, National Yang-Ming University, Taipei, Taiwan, 9Integrated Brain Research Unit, Department of Medical Research, Taipei Veterans General Hospital, Taipei, Taiwan, 10Institute of Biomedical Informatics, National Yang-Ming University, Taipei, Taiwan
Synopsis
The contributions of blood vessels impairment to dementia are increasingly recognized, but the relationships between microbleeds and blood–brain barrier (BBB) in dementia patients are still unclear. We recruited subjects with Alzheimer’s disease (AD), mild cognitive impairment (MCI), subjective cognitive decline (SCD), and healthy control (HC). The susceptibility weighted images and dynamic contrast-enhanced images were acquired to detect cerebral microbleeds and to estimate BBB integrity. Our results showed high risk with BBB breakdown in AD patients with cerebral microbleeds, which suggests the association between neurovascular unit dysfunction and blood vessel impairment may be a biomarker for the progression of dementia.
Introduction
Vascular damage such as cerebral microbleeds may be associated with blood-brain barrier (BBB) breakdown, which leads to neuronal injury and increased neurodegenerative risk. However, the associations between cerebral microbleeds and BBB permeability in different severity of dementia, including the initial stage of subjective cognitive decline (SCD) complaints, follows by mild cognitive impairment (MCI) and Alzheimer’s disease (AD) later, remain elusive. The present study aimed to investigate the relationship between microbleeds and regional BBB permeability in patients with different severity of dementia.Methods
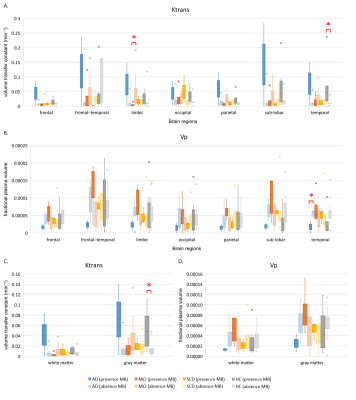
We recruited fifty-one subjects, including ten AD, fourteen MCI, eight SCD patients, and nineteen healthy control (HC) subjects. All subjects received neuropsychological testing and PET/MR scans using a 3T PET/MR scanner (Signa, GE Healthcare, Milwaukee, USA) at Taipei Veterans General Hospital. Different MR images including T1-weighted anatomic image, susceptibility weighted image (SWI) and dynamic contrast-enhanced MRI (DCE-MRI) were acquired. We used a homemade user interface to locate the cerebral microbleeds, which were defined as homogeneous near-round focal areas < 10 mm in diameter of low signal intensity on SWI, for each subject. The DCE-MRI were analyzed using the Medical Imaging Interaction Toolkit (MITK) toolbox1. Individual arterial input functions were extracted from the anterior cerebral artery. We adopted the extended tofts model to estimate the features of BBB permeability, including volume transfer constant (Ktrans), reflux rate (Kep), fractional extravascular extracellular space volume (Ve), and fractional plasma volume (Vp), followed by regions of interest (ROI) analysis. We selected 7 brain regions (frontal, frontal-temporal, limbic, occipital, parietal, sub-lobar, and temporal lobe) from the Talairach Daemon Labels using WFU_Pickatlas toolbox as will as the regions of gray matter and white matter in the whole brain obtained from individual segmented T1-weighted image using SPM 12. To investigate the association between appearance of microbleeds and BBB permeability, we partitioned the subjects into subgroups with presence or absence of microbleeds. The number of presence or absence of microbleeds in each group was as follows (presence/absence): AD (2/8), MCI (4/10), SCD (3/5), HC (6/13). Mann-Whitney U test was performed to compare the indices of BBB permeability between groups. The results were considered to be significant at p < 0.05.Results
We found that AD patients with microbleeds had lower fractional plasma volume (Vp) value in the temporal lobe compared to AD patients without microbleeds. Moreover, the AD patients with microbleeds showed higher volume transfer constant (Ktrans) and lower fractional plasma volume (Vp) values compared to AD patients without microbleeds, although there were no significant differences in each ROI. The MCI patients with microbleeds exhibited lower volume transfer constant (Ktrans) value in the limbic lobe compared to MCI patients without microbleeds. In the control group, the subjects with microbleeds had higher volume transfer constant (Ktrans) value in temporal lobe and gray matter than subjects without microbleeds.Discussion
The present study showed higher volume transfer constant (Ktrans) and lower plasma volume (Vp) in AD patients with microbleeds, compared to AD patients without microbleed. In the subjects with microbleeds, AD had lower fractional plasma volume than HC in the white matter and gray matter, indicating hypoperfusion in AD; the volume transfer constant was higher in AD compared to HC in the gray matter and cortex, indicating global BBB leakage in AD2. The present study demonstrated that the presence of cerebral microbleeds was in a high risk with BBB breakdown, which reflect in high BBB leakage rate and blood hypoperfusion. BBB permeability is regulated in the neurovascular unit. BBB breakdown may imply the dysfunction of neurovascular unit3, which might be the etiology of dementia. Our findings may provide a potential insight to further neurovascular unit function in patients with different severity of dementia.Acknowledgements
This work was supported in part by the Taiwan Ministry of Science and Technology (MOST 107-2221-E-010-013, MOST107-2221-E075-006, MOST107-2221-E010-014), the Brain Research Center of National Yang-Ming University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan, and Academia Sinica.References
1. Debus, C., Floca, R., Ingrisch, M., et al. (2019). MITK-ModelFit: A generic open-source framework for model fits and their exploration in medical imaging - design, implementation and application on the example of DCE-MRI. BMC Bioinformatics, 20(1), 31.
2. Van De Haar, H. J., Burgmans, S., Jansen, J. F., et al. (2016). Blood-brain barrier leakage in patients with early Alzheimer disease. Radiology, 281(2), 527-535.
3. Zlokovic, B. V. (2011). Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci, 12(12), 723-738.
Figures