1684
Mechanical response of the brain to shunt placement in normal pressure hydrocephalus1Mayo Clinic, Rochester, MN, United States
Synopsis
Normal pressure hydrocephalus (NPH) is a neurological disorder characterized by abnormal gait, cognitive decline, and urinary incontinence. The hypothesized role of biomechanics in NPH pathogenesis supports a potential role for magnetic resonance elastography (MRE) in diagnosis and prediction of response to therapy. In this study, MRE was performed in 13 NPH patients before and after shunt placement to test the hypothesis that treatment would reverse NPH-driven changes to the brain’s mechanical properties. We observed that increased stiffness and decreased damping ratio at the vertex were largely reversed by shunting, while periventricular white matter softening was unaffected.
Introduction
Normal pressure hydrocephalus (NPH) is a neurological disorder characterized by abnormal gait, cognitive decline, and urinary incontinence.1 Novel biomarkers to aid early and accurate diagnosis of NPH would be significant because, unlike other forms of dementia, symptoms due to NPH are potentially treatable by shunt placement. In particular, an effective biomarker would differentiate NPH from other causes of enlarged cerebrospinal fluid spaces (e.g., Alzheimer’s disease) and also predict response to shunting. Given the hypothesized role of biomechanics in NPH pathogenesis,2-4 initial studies of NPH using magnetic resonance elastography (MRE) have been performed, indicating a disease-specific pattern of mechanical property changes.5-8 In this study, we tested the hypothesis that these mechanical alterations would be reversed by shunt placement in patients shown to improve clinically.Methods
Thirteen NPH patients (aged 75.1 ± 5.5 years, mean ± standard deviation) underwent MRE exams both before and after shunt placement after obtaining IRB approval and informed written consent. MRE data were collected with a single-shot spin-echo EPI pulse sequence using 60 Hz vibration and a final image resolution of 3 mm isotropic, as previously described.9 T1-weighted images acquired in the same exam were used for brain segmentation and to define regions of interest using an in-house template and atlas by unified segmentation in SPM.10, 11 An edge-aware neural network inversion (NNI) was used to estimate stiffness and damping ratio maps for each MRE exam.12 This inversion had a 7×7×7 voxel footprint, and was trained under the assumption of homogeneous material properties. Using the previously computed deformation fields, the mechanical property maps were warped to template space to allow voxel-wise modeling. To test the above hypothesis, a paired t-test was performed to compare the mechanical property maps before and after shunting. For each mechanical property, a family-wise error corrected P<0.025 was considered significant to maintain an overall 5% false positive rate using approximate permutation tests (1,000 permutations). To aid interpretation, the NPH participants both pre- and post-shunting were also compared against an age-matched (74.5 ± 9.3 years) amyloid-negative cognitively unimpaired (CU) group by computing mean difference maps for each mechanical property.13Results
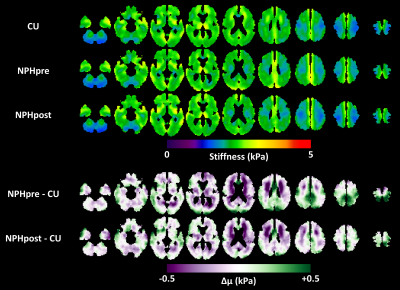
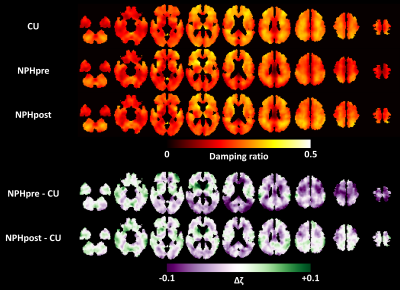
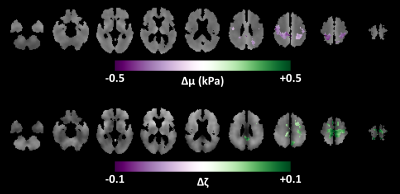
Figure 1 shows the mean stiffness maps for the CU participants and the NPH patients both pre- and post-shunt placement, as well as the mean difference in stiffness between the two groups. As previously reported, stiffness alterations occur in a concentric pattern including periventricular softening and stiffening near the dural surface, particular at the vertex and in the occipital lobe. Analogous results for damping ratio are shown in Figure 2. Damping ratio is mostly reduced, particularly beginning at the level of the lateral ventricles and superior. The difference in mechanical properties before and after shunt placement is summarized in Figure 3. First considering stiffness changes, two significant clusters were found near the vertex in which stiffness was significantly reduced post-shunt. Considering damping ratio, one significant cluster (spanning both hemispheres) was identified, also at the vertex, in which the damping ratio was increased. Both of these changes made the mechanical properties more similar to the CU group in these particular regions, though the values did not fully return to those observed in CU participants. On the other hand, no significant changes in stiffness or damping ratio were observed in the periventricular white matter. Furthermore, Figures 1 and 2 show no obvious alterations in the periventricular white matter in the NPH group following shunt placement.Discussion and conclusions
In general, NPH-driven mechanical changes near the vertex of the brain were largely reversed following shunt placement in these patients, each of whom improved clinically. This reversal suggests that mechanical alterations at the vertex represent a mass effect, where the compression on the brain parenchyma causes increased stiffness due to the nonlinear elastic behavior of brain tissue,14 as well as decreased viscosity. If we consider brain parenchyma as a poroelastic material, this latter observation may be explained as the removal of interstitial fluid due to NPH-driven compression that can be mitigated by shunt placement. On the other hand, the periventricular softening due to NPH was unaltered by shunt placement, suggesting an irreversible degenerative process. Freimann et al. previously used MRE to measure the mechanical properties in the brain of NPH patients before and after shunt placement.6 They reported an increase in the springpot parameter, α, which is consistent with our finding of increased damping ratio. However, they found no change in stiffness. This discrepancy likely arises from ROI selection since they examined one slice centered on the lateral ventricles, while in this study we have found most alterations occurring at the vertex. Taken together with previous studies, this work provides further support for evaluating MRE as a practical clinical tool for noninvasive diagnosis, shunting prognosis, and monitoring of NPH.Acknowledgements
No acknowledgement found.References
1. Adams RD, Fisher CM, Hakim S, Ojemann RG, Sweet WH. Symptomatic Occult Hydrocephalus with "Normal" Cerebrospinal-Fluid Pressure.A Treatable Syndrome. The New England journal of medicine. 1965;273:117-26. doi: 10.1056/NEJM196507152730301. PubMed PMID: 14303656.
2. Hakim S, Adams RD. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure. Observations on cerebrospinal fluid hydrodynamics. Journal of the neurological sciences. 1965;2(4):307-27. PubMed PMID: 5889177.
3. Hakim S, Venegas JG, Burton JD. The physics of the cranial cavity, hydrocephalus and normal pressure hydrocephalus: mechanical interpretation and mathematical model. Surg Neurol. 1976;5(3):187-210. PubMed PMID: 1257894.
4. Keong NC, Pena A, Price SJ, Czosnyka M, Czosnyka Z, Pickard JD. Imaging normal pressure hydrocephalus: theories, techniques, and challenges. Neurosurgical focus. 2016;41(3):E11. doi: 10.3171/2016.7.FOCUS16194. PubMed PMID: 27581307.
5. Fattahi N, Arani A, Perry A, Meyer F, Manduca A, Glaser K, Senjem ML, Ehman RL, Huston J. MR Elastography Demonstrates Increased Brain Stiffness in Normal Pressure Hydrocephalus. AJNR American journal of neuroradiology. 2016;37(3):462-7. doi: 10.3174/ajnr.A4560. PubMed PMID: 26542235; PMCID: 4792773.
6. Freimann FB, Streitberger KJ, Klatt D, Lin K, McLaughlin J, Braun J, Sprung C, Sack I. Alteration of brain viscoelasticity after shunt treatment in normal pressure hydrocephalus. Neuroradiology. 2012;54(3):189-96. doi: 10.1007/s00234-011-0871-1. PubMed PMID: 21538046.
7. Perry A, Graffeo CS, Fattahi N, ElSheikh MM, Cray N, Arani A, Ehman RL, Glaser KJ, Manduca A, Meyer FB, Huston J, 3rd. Clinical Correlation of Abnormal Findings on Magnetic Resonance Elastography in Idiopathic Normal Pressure Hydrocephalus. World neurosurgery. 2017;99:695-700 e1. doi: 10.1016/j.wneu.2016.12.121. PubMed PMID: 28063896; PMCID: 5357459.
8. Streitberger KJ, Wiener E, Hoffmann J, Freimann FB, Klatt D, Braun J, Lin K, McLaughlin J, Sprung C, Klingebiel R, Sack I. In vivo viscoelastic properties of the brain in normal pressure hydrocephalus. NMR in biomedicine. 2011;24(4):385-92. Epub 2010/10/12. doi: 10.1002/nbm.1602. PubMed PMID: 20931563.
9. Murphy MC, Huston J, 3rd, Jack CR, Jr., Glaser KJ, Senjem ML, Chen J, Manduca A, Felmlee JP, Ehman RL. Measuring the characteristic topography of brain stiffness with magnetic resonance elastography. PloS one. 2013;8(12):e81668. doi: 10.1371/journal.pone.0081668. PubMed PMID: 24312570; PMCID: 3847077.
10. Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26(3):839-51.
11. Vemuri P, Gunter JL, Senjem ML, Whitwell JL, Kantarci K, Knopman DS, Boeve BF, Petersen RC, Jack CR, Jr. Alzheimer's disease diagnosis in individual subjects using structural MR images: validation studies. NeuroImage. 2008;39(3):1186-97. doi: 10.1016/j.neuroimage.2007.09.073. PubMed PMID: 18054253; PMCID: 2390889.
12. Murphy MC, Manduca A, Trzasko JD, Glaser KJ, Huston J, 3rd, Ehman RL. Artificial neural networks for stiffness estimation in magnetic resonance elastography. Magnetic resonance in medicine. 2018;80(1):351-60. doi: 10.1002/mrm.27019. PubMed PMID: 29193306; PMCID: 5876084.
13. Arani A, Murphy MC, Glaser KJ, Manduca A, Lake DS, Kruse SA, Jack CR, Jr., Ehman RL, Huston J, 3rd. Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults. NeuroImage. 2015;111:59-64. doi: 10.1016/j.neuroimage.2015.02.016. PubMed PMID: 25698157; PMCID: 4387012.
14. Arani A, Min HK, Fattahi N, Wetjen NM, Trzasko JD, Manduca A, Jack CR, Jr., Lee KH, Ehman RL, Huston J, 3rd. Acute pressure changes in the brain are correlated with MR elastography stiffness measurements: initial feasibility in an in vivo large animal model. Magnetic resonance in medicine. 2018;79(2):1043-51. Epub 2017/05/11. doi: 10.1002/mrm.26738. PubMed PMID: 28488326; PMCID: PMC5811891.
Figures