1683
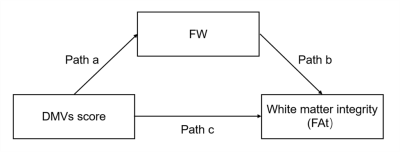
Venous disruption affects white matter integrity through increased interstitial fluid in cerebral small vessel disease1The Second Affiliated Hospital of Zhejiang University, School of Medicine, Hangzhou, China
Synopsis
We aimed to investigate whether and how venous disruption was related to white matter damage in cerebral small vessel disease(CSVD) patients. SWI was used to investigate venous disruption. Free water elimination DTI model was used to analyze interstitial fluid fraction(fraction of free water, fFW) and white matter integrity(tissue fractional anisotropy, FAt). In 104 CSVD patients, venous disruption was associated with FAt, and the effect was mediated by fFW. This relationship was independent of arterial cerebral blood flow. In conclusion, we discovered the venous disruption - increased interstitial fluid - white matter damage link in CSVD patients.
Introduction
White matter damage is common in Cerebral small vessel disease (CSVD) and is associated with cognitive impairment,1 gait2 and mood disorders.3 Although arterial perfusion could affect white matter integrity,4 it was also shown that venous disruption might play a role.5Deep medullary veins (DMVs) drain their surrounding white matter.6 Pathological and imaging studies found DMVs disruption was related to white matter hyperintensities (WMH).7-9 However, WMH cannot accurately represent white matter damage due to its heterogeneous histopathology.10 And white matter damage in CSVD is not confined to WMH regions.11 Analyzing microstructural changes of white matter in DMVs drainage area with advanced imaging techniques is necessary.
Moreover, the potential pathway of how DMVs disruption affect white matter remains unclear. Previous studies assumed that diminished venous outflow could lead to increased interstitial fluid, which may cause metabolic waste aggregation and inflammation, finally resulting in white matter damage.12 However, these theories remained as hypotheses without evaluation in CSVD patients or animal models.
Therefore, in the present study, we aimed to investigate the relationship between DMVs disruption and white matter damage, and to explore whether this link is mediated by increased interstitial fluid through a comprehensive multi-modality MRI study.
Methods
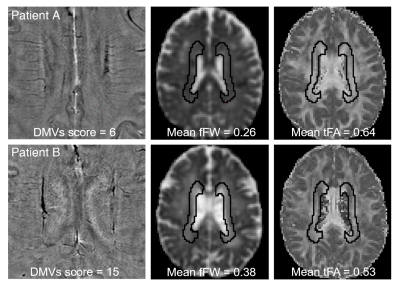
We retrospectively reviewed the clinical, laboratory and imaging data of CSVD patients admitted to the neurology department of our hospital. A hundred and four CSVD patients with complete clinical data and multi-modality MRI data were included. The susceptibility weighted imaging (SWI) phase images were used to observe the morphological characteristics of DMVs, based on which the brain region-based DMVs visual scores were given.9 Free water (FW) elimination diffusion tensor imaging (DTI) model was used to analyze the interstitial fluid fraction (fraction of free water, fFW) and white matter integrity (tissue fractional anisotropy, FAt).13, 14 Mediation analyses were used to examine the potential relationship between DMVs score, fFW and FAt of the DMVs drainage regions. To exclude the possible effect of arterial perfusion on white matter integrity, we also performed a second mediation analysis controlling for regional cerebral blood flow (CBF).Results
The total DMVs score was associated with FAt of DMVs drainage area in CSVD patients (Pearson r = -0.381, p < 0.001) after adjusting for age and gender. FAt of each subregion correlated with the DMVs score of the same subregion, respectively. The effect of total DMVs score on FAt was mediated by fFW (direct effects: std β = -0.129, p > 0.05; indirect effects: std β = -0.243, p < 0.05). After adjusting for CBF, the mediation effect remained significant (direct effects: std β = -0.127, p > 0.05; indirect effects: std β = -0.238, p < 0.05). The relationships between DMVs score, fFW and FAt were also significant in the six DMVs drainage subregions.Discussion
In the current study, we found that DMVs score was associated with FAt in CSVD patients, and this effect was mediated by fFW. Venous disruption related white matter damage in CSVD patients had been proposed since Moody et al. discovered venous collagenosis in WMH areas in 1995.7 Venous collagenosis could lead to intramural thickening, stenosis and ultimately luminal occlusion, causing the elevation of venous pressure.5 Animal study discovered that venous hypertension could lead to neuronal degeneration and loss of white matter integrity.15 Consistently, our imaging study found that DMVs score was associated with FAt, demonstrating the connection between venous disruption and loss of white matter integrity in CSVD patients. Notably, our study demonstrated the mediation role of fFW between DMVs score and white matter integrity. Previous studies demonstrated that elevation of fFW was associated with vasogenic edema within the brain parenchyma in the extracellular space due to processes such as ischemic stroke and tumors.13, 16 In CSVD patients, DMVs disruption could result in the resistance to cerebrospinal fluid absorption and increased fluid leakage into the perivascular space, leading to interstitial edema. Previous studies on cerebral venous thrombosis also found that venous occlusion could lead to brain edema,17 providing evidence for our hypothesis. In addition, our study found that the relationship between DMVs score, fFW and FAt was independent of CBF. Low CBF has been demonstrated to be related with atherosclerosis and could lead to white matter damage.18 A recent study discovered that elevated fFW and decreased FAt was related to arterial stiffness.19 Our study demonstrated the venous side of hemodynamic alteration related extracellular water increase and white matter damage.Conclusion
We discovered the DMVs disruption - increased interstitial fluid - white matter damage link in CSVD patients, which was independent of arterial perfusion variations. Our findings might provide insights into new therapies for CSVD related white matter damage.Acknowledgements
No acknowledgement found.References
1. Ter Telgte, A., E.M.C. van Leijsen, K. Wiegertjes, et al., Cerebral small vessel disease: from a focal to a global perspective. Nat Rev Neurol, 2018; 14(7): 387-398.
2. de Laat, K.F., A.M. Tuladhar, A.G.W. van Norden, et al., Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain, 2011; 134: 73-83.
3. Rensma, S.P., T.T. van Sloten, L.J. Launer, et al., Cerebral small vessel disease and risk of incident stroke, dementia and depression, and all-cause mortality: A systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews, 2018; 90: 164-173.
4. Promjunyakul, N.O., H.H. Dodge, D. Lahna, et al., Baseline NAWM structural integrity and CBF predict periventricular WMH expansion over time. Neurology, 2018; 90(24): e2119-e2126.
5. Keith, J., F.Q. Gao, R. Noor, et al., Collagenosis of the Deep Medullary Veins: An Underrecognized Pathologic Correlate of White Matter Hyperintensities and Periventricular Infarction? J Neuropathol Exp Neurol, 2017; 76(4): 299-312.
6. Taoka, T., A. Fukusumi, T. Miyasaka, et al., Structure of the Medullary Veins of the Cerebral Hemisphere and Related Disorders. Radiographics, 2017; 37(1): 281-297.
7. Moody, D.M., W.R. Brown, V.R. Challa, et al., Periventricular venous collagenosis: association with leukoaraiosis. Radiology, 1995; 194(2): 469-76.
8. Brown, W.R., D.M. Moody, V.R. Challa, et al., Venous collagenosis and arteriolar tortuosity in leukoaraiosis. J Neurol Sci, 2002; 203-204: 159-63.
9. Zhang, R., Y. Zhou, S. Yan, et al., A Brain Region-Based Deep Medullary Veins Visual Score on Susceptibility Weighted Imaging. Frontiers in Aging Neuroscience, 2017; 9: 269.
10. Gouw, A.A., A. Seewann, W.M. van der Flier, et al., Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry, 2011; 82(2): 126-35.
11. Zhong, G. and M. Lou, Multimodal imaging findings in normal-appearing white matter of leucoaraiosis: a review. Stroke Vasc Neurol, 2016; 1(2): 59-63.
12. Black, S., F. Gao, and J. Bilbao, Understanding white matter disease: imaging-pathological correlations in vascular cognitive impairment. Stroke, 2009; 40(3 Suppl): S48-52.
13. Pasternak, O., N. Sochen, Y. Gur, et al., Free water elimination and mapping from diffusion MRI. Magn Reson Med, 2009; 62(3): 717-30.
14. Duering, M., S. Finsterwalder, E. Baykara, et al., Free water determines diffusion alterations and clinical status in cerebral small vessel disease. Alzheimers & Dementia, 2018; 14(6): 764-774.
15. Zhang, H.Q., T. Chen, S.S. Wu, et al., The pathophysiology of venous hypertensive myelopathy--study of an animal model: laboratory investigation. J Neurosurg Spine, 2013; 19(4): 485-91.
16. Duering, M., R. Adam, F.A. Wollenweber, et al., Within-lesion heterogeneity of subcortical DWI lesion evolution, and stroke outcome: A voxel-based analysis. J Cereb Blood Flow Metab, 2019: 271678X19865916.
17. Zuurbier, S.M., R. van den Berg, D. Troost, et al., Hydrocephalus in cerebral venous thrombosis. J Neurol, 2015; 262(4): 931-7.
18. Badji, A., D. Sabra, L. Bherer, et al., Arterial stiffness and brain integrity: A review of MRI findings. Ageing Res Rev, 2019; 53: 100907.
19. Maillard, P., G.F. Mitchell, J.J. Himali, et al., Aortic Stiffness, Increased White Matter Free Water, and Altered Microstructural Integrity: A Continuum of Injury. Stroke, 2017; 48(6): 1567-1573.
Figures