1677
Choroid plexus and gray matter perfusion dissimilarity in patients with cerebrovascular disease: implications for CSF dynamics and glymphatics1Radiology and Radiological Sciences, Vanderbilt University School of Medicine, Nashville, TN, United States, 2Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, United States, 3Neurology, Vanderbilt University School of Medicine, Nashville, TN, United States, 4Psychiatry, Vanderbilt University School of Medicine, Nashville, TN, United States
Synopsis
The goal of this study is to investigate whether choroid plexus (CP) activity, quantified as perfusion measured from arterial spin labeling, is altered in patients with cerebrovascular disease. Cohorts comprised arterial intracranial stenosis (n=56), sickle cell disease (n=41), and healthy controls (n=31). In controls and stenosis patients, CP and gray matter perfusion were similar; in patients with sickle cell disease, CP-to-gray matter perfusion was reduced (p<0.001) relative to other cohorts. This finding is discussed in the context of how efficient CSF perivascular flow surrounding dilated arterial spaces may require reduced CSF production activity to subserve waste clearance.
Introduction
The overall goal of this study is to investigate whether choroid plexus (CP) activity, quantified as perfusion measured from non-invasive arterial spin labeling (ASL), is altered in patients with cerebrovascular disease of differing etiology.The CP is responsible for the production of CSF and also represents one locus of exchange of blood and cerebrospinal fluid (CSF) at the blood-CSF barrier1. As such, CP activity may have relevance to CSF production activity2, circulating markers of neuronal stress3, and has been shown to decrease following surgically-induced angiogenesis of the cortex in humans4, which may be indirectly attributed to improved perivascular flow: a central component of the recently proposed, controversial glymphatic system5. More specifically, the CP complexes receive arterial supply from the choroidal arteries, which arise from distal branches of the internal carotid arteries or branches of the posterior cerebral arteries6. Activity of these complexes is not routinely measured, and perfusion signal in the lateral ventricles is frequently attributed to CSF pulsatility artifact. Given that the arterial supply of the CP is derived from arteries distal to those labeled in typical arterial spin labeling (ASL) planes, we (1) performed repeated ASL measurements with differing labeling parameters and quantified CP perfusion and reproducibility in healthy controls, and (2) repeated measures in patients with cerebrovascular disease of differing etiology including those (i) with intracranial arterial stenosis or (ii) without arterial stenosis but with dilated intracranial arterioles secondary to reduced oxygen carrying capacity related to sickle cell disease. The hypothesis to be investigated is that CP perfusion measured from ASL is reproducible and is similar to gray matter perfusion; furthermore, given its potential relevance as a centralized marker of neuronal stress, CP perfusion is increased in cerebrovascular disease patients with both low (secondary to steno-occlusion) and high (secondary to anemia) cortical perfusion.Method
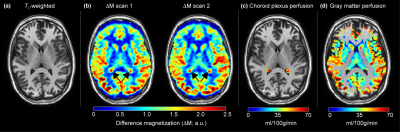
Experiment. All participants (n=128) provided informed, written consent for this cross-sectional 3T (Philips Healthcare, Best, The Netherlands) study. Cohorts comprised (i) intracranial arterial stenosis (n=56), (ii) sickle cell disease (n=41), and (iii) healthy control (n=31) participants. A subset of healthy controls (n=10) were scanned twice for reproducibility. MRI. Standard anatomical imaging, time-of-flight angiography, and whole-brain pseudo-continuous ASL (pCASL) approaches covering the CP complexes and supratentorial gray matter were applied from two protocols (Protocol-1: labeling duration=1650 ms, post-labeling delay=2000 ms; Protocol-2: labeling duration=1000 ms, post-labeling delay=1900 ms). Analysis. Perfusion was quantified from pCASL data and calculated in the CP located in the atrium of the lateral ventricles, as identified by T1-weighted MRI, and in the total gray matter, calculated from FSL-FAST (Figure 1). Statistical analysis. For reproducibility assessment, perfusion from repeated scans was calculated in control volunteers for each set of scan parameters and compared using a paired Student’s t-test, intraclass correlation (ICC), and Pearson’s correlation test. Next, mean CP perfusion, gray matter perfusion, and the ratio of CP-to-gray matter perfusion between the three cohorts were compared using an unpaired t-test. In all cases, two-sided p<0.05 was required for significance.Results
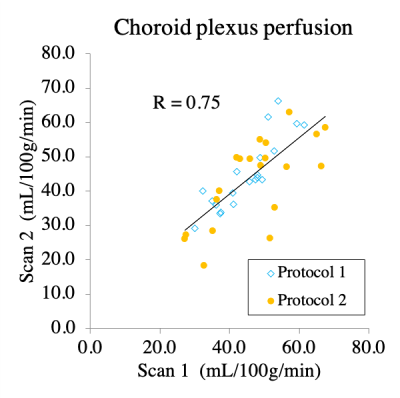
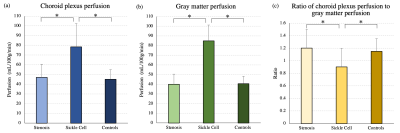
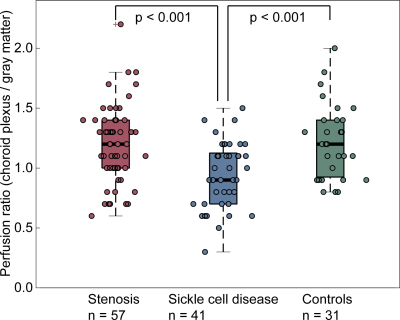
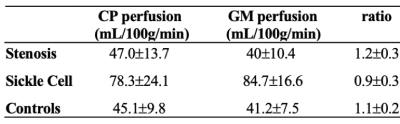
CP perfusion values between scans for pCASL methods for both protocols were found not to be significantly different: Protocol-1 scan-1=44.9±8.1 ml/100g/min and scan-2=44.9±9.9 ml/100g/min; p=0.98; Protocol-2 scan-1=47.2±11.9 ml/100g/min and scan-2=43.3±11.0 ml/100g/min; p=0.12 (Figure 2).The CP-to-gray matter perfusion ratio was similar at 1.2±0.1 and 1.1±0.3 for Protocol-1 and Protocol-2, respectively. The ICC for CP and gray matter perfusion were both 0.69, consistent with moderate-to-good agreement in both regions.In intracranial arterial stenosis patients with regionally reduced cortical perfusion, there was slightly elevated CP perfusion (47.0±13.7 ml/100g/min) compared to gray matter perfusion (40±10.4 ml/100g/min), resulting in a CP to gray matter perfusion ratio of 1.2±0.3 (Figure 3). In sickle cell disease patients with reduced oxygen carrying capacity secondary to anemia, but without large vessel arterial steno-occlusion, there was elevated gray matter perfusion (84.7±16.6 ml/100g/min) as expected to offset reduced oxygen carrying capacity, but this mean gray matter perfusion was higher compared to CP perfusion (78.3±24.1 ml/100g/min), resulting in a lower CP-to-gray matter perfusion ratio (ratio=0.9±0.3). This ratio was statistically reduced compared to intracranial arterial stenosis (p<0.001) and control (p<0.001) participants (Figure 4; Figure 5).Discussion and Conclusion
In those with arterial steno-occlusion, CP perfusion and gray matter perfusion were similar, and the ratio of perfusion values was mildly, but not significantly, elevated relative to control values. In patients with sickle cell disease, CP perfusion was reduced relative to gray matter perfusion and this ratio was significantly lower relative to controls and arterial steno-occlusion patients. These findings are not consistent with the primary hypothesis that CP perfusion provides a central indicator of ischemia and associated circulating markers of neuronal stress. An alternate explanation is that in patients with sickle cell disease, increased cerebral blood flow promotes increased arterial pulsitility, which drives robust arterial perivascular flow. As such, glymphatic waste clearance is enhanced and the requirement of CP perfusion and CSF production to support waste clearance is reduced, consistent with recently proposed glymphatic clearance mechanisms5. An additional consideration is that endovascular signal artifact present on arterial steno-occlusion patient data may artifactually overestimate cortical perfusion when measured from ASL7, 8; as cervical stenosis and posterior circulation stenosis was not present in these patients, CP perfusion should suffer less from this possible confound in these patients.Acknowledgements
No acknowledgement found.References
1. Sakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. European annals of otorhinolaryngology, head and neck diseases 2011;128:309-316.
2. Maktabi MA, Stachovic GC, Faraci FM. Angiotensin II decreases the rate of production of cerebrospinal fluid. Brain research 1993;606:44-49.
3. Emerich DF, Vasconcellos AV, Elliott RB, Skinner SJ, Borlongan CV. The choroid plexus: function, pathology and therapeutic potential of its transplantation. Expert Opin Biol Ther 2004;4:1191-1201.
4. Johnson SE, McKnight CD, Lants SK, et al. Choroid plexus perfusion and intracranial cerebrospinal fluid changes after angiogenesis. J Cereb Blood Flow Metab 2019:271678X19872563.
5. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Science translational medicine 2012;4:147ra111.
6. Wiesmann M, Yousry I, Seelos KC, Yousry TA. Identification and anatomic description of the anterior choroidal artery by use of 3D-TOF source and 3D-CISS MR imaging. AJNR Am J Neuroradiol 2001;22:305-310.
7. Fan AP, Jahanian H, Holdsworth SJ, Zaharchuk G. Comparison of cerebral blood flow measurement with [15O]-water positron emission tomography and arterial spin labeling magnetic resonance imaging: A systematic review. J Cereb Blood Flow Metab 2016;36:842-861.
8. Mutsaerts HJ, Petr J, Vaclavu L, et al. The spatial coefficient of variation in arterial spin labeling cerebral blood flow images. J Cereb Blood Flow Metab 2017;37:3184-3192.
Figures