1655
Multi-modal assessment of cerebrovascular reactivity by multiband BOLD and multidelay ASL in moyamoya patients using acetazolamide1Neurosurgery, UMC Utrecht, Utrecht, Netherlands, 2Radiology, UMC Utrecht, Utrecht, Netherlands, 3Pediatric Neurology, UMC Utrecht, Utrecht, Netherlands
Synopsis
Measuring the cerebral blood flow and cerebrovascular reactivity is vital in the preoperative assessment of moyamoya patients. We propose a combination of continuous multiband BOLD-MRI and multidelay ASL-MRI with acetazolamide as a comprehensive multi-modal alternative for the current commonly used PET-CT method. Eight female patients (age 8-41) were scanned at 3T with a ±30 min scan protocol, which is also suitable for patients scanned under general anesthesia. The obtained reactivity maps of the two sequences showed good agreement for severity and location of hemodynamic impairment. This accessible MRI method is directly suitable for routine clinical evaluation of moyamoya patients.
Introduction
The assessment of cerebrovascular hemodynamics is essential in the preoperative diagnostics and evaluation of patients with moyamoya vasculopathy (MMV)1–3. Cerebral blood flow (CBF) and cerebrovascular reactivity (CVR) obtained with a H215O PET-CT combined with an acetazolamide (ACZ) vascular challenge are considered the gold standard2,4. However, this method has several drawbacks: (1) limited availability (2) ionizing radiation, (3) high costs and (4) added patient burden. Multi-modal hemodynamic assessment with BOLD and ASL-MRI can offer a widely available, minimally invasive and lower-cost alternative for obtaining CBF and CVR values5,6. Standard, single post label delay (PLD) ASL is sensitive to long arterial transit times (ATT) characteristic of MMV pathology7. This limitation can be reduced by employing multiple PLD times (multidelay; MD) to sensitize for perfusion in long ATT areas3. Whereas BOLD is unsuitable for assessment of baseline CBF, its relative measure is insensitive to long ATT providing extra information in addition to ASL8,9. The high signal contrast-to-noise ratio and temporal resolution of multiband BOLD imaging can capture dynamic signal changes to which ASL is insensitive. However, hemodynamic assessments using either modality have been shown to be comparable to the gold standard2,10–12. Still, no standardized clinical protocol has been established. Working towards this, we started with a minimally-invasive and pragmatic approach of combining BOLD and ASL with ACZ administration (figure 1) along with standard clinical scans (MRA, DWI, FLAIR if indicated), to extract additional quantitative and qualitative information on CVR/CBF within one MRI slot. Our study was primarily aimed at confirming the practicality of this simple setup in a routine clinical environment. Second to this, we acquired insight on the dynamic stimulus effect of ACZ on the BOLD signal during and after injection. To the best of knowledge this has not been shown before in MMV.Methods
We reviewed all consecutive patients who had undergone a new (preliminary) clinical protocol for cerebrovascular hemodynamic evaluation in MMV in our center, starting October 2018. All scans were performed on a 3T MRI system (Philips, Best, the Netherlands). Baseline CBF measurements were obtained using an efficient Look-Locker MD-ASL approach: 5 PLDs (1206-3480ms), pCASL, multi-slice EPI, label duration=2s, voxelsize=3.75x3.75x7mm3,16 slices, FOV=240x240x120mm3, TR/TE=6s/11ms, flip angle=25°, SENSE factor=2, 4 background suppression, 24 volumes, scantime=5min. The ACZ challenge consisted of 20mg/kg ACZ (max 1000mg), injected 1 minute after initialization of continuous multiband-BOLD scanning; multi-slice gradient-echo EPI, voxelsize=2.5mm isotropic, 48 slices, FOV=224x224x120mm3, TR/TE=1.1s/35ms, flip angle=65° SENSE factor=1.7, multiband factor=3, 825 volumes, scan time=15.5min. 12 minutes after injection the MD-ASL was repeated for comparison with the pre-ACZ scan and calculation of CVR. Scans were qualitatively assessed for image quality, and perfusion and CVR deficits for clinical guidance. BOLD data was motion-corrected (FSL (FMBRIB, Oxford, UK)) and spatial (5mm, 3D Gaussian kernel) and temporal smoothing was applied (LOESS filter, 6% regression window). Mean BOLD images were segmented and the average gray matter (GM) time-series was used as a regressor to evaluate voxel-wise correlation co-efficient. Positive correlations represented signal increases in response to ACZ (indicating positive CVR) while negative correlations suggested the presence of vascular steal. MD-ASL data were analyzed using the BASIL tool (FSL), which provides absolute CBF and estimated ATT maps.Results
8 patients were identified and retrospectively reviewed (table 1). Data from all patients proved useable for clinical evaluation. In all patients hemodynamic impairment was identified and the location and size of the deficit was similar between ASL and BOLD. In two subjects a recent PET (<2 months apart) was available, which provided similar qualitative results (see figure 2). See figure 3 for an example of all information from the protocol in one patient. The multiband-BOLD shows the dynamic ACZ effect: the CBF steadily increases for the healthy regions until a plateau is reached (with 2.5% BOLD-increase in this example). For regions with impaired CVR <30s after injection of ACZ a transient decrease in CBF (vascular steal) is seen, which rapidly (around 160s after injection) plateaus.Discussion
This study shows that qualitative hemodynamic assessment on CBF and CVR using a multi-modal approach with BOLD and ASL in combination with ACZ is a robust method that provides valuable pre- and postoperative information in MMV. Using this multi-modal assessment a decision for revascularization surgery was made in 3 cases. The MD-ASL scan is less sensitive to long ATT, whereas with the use of the BOLD scan the effect of ACZ can be continuously monitored. The combined ASL and BOLD methods provide complementary information for an accurate hemodynamic assessment. The earlier plateau-phase for impaired region (figure 3) could be explained by the sigmoidal shape of the vascular response to the effects of ACZ13,14, as shown and explained in figure 4. Although it is readily affordable and simple to use, ACZ has its drawbacks, like the need of the injection itself and the required clinical observation. During this observation two patients experiences headaches in this study, necessitating prolonging of stay in one patient.Conclusion
Multi-modal hemodynamic assessment using BOLD and ASL MRI offers a robust alternative to the PET-CT in MMV. MD-ASL gives baseline CBF and postACZ CBF information producing a CVR map similar to PET-CT. The BOLD offers complementary dynamic information on the induced ACZ hemodynamic changes.Acknowledgements
We thank our colleague Annick Kronenburg as member of the moyamoya vasculopathy research team for the provided insight and expertise.References
1. Kronenburg
A, Braun KPJ, Van Der Zwan A, Klijn CJM. Recent advances in moyamoya disease: Pathophysiology and treatment. Curr
Neurol Neurosci Rep. 2014;14(1):1-9. doi:10.1007/s11910-013-0423-7.
2. Fierstra J,
van Niftrik C, Warnock G, et al. Staging Hemodynamic
Failure With Blood Oxygen-Level-Dependent Functional Magnetic Resonance Imaging
Cerebrovascular Reactivity: A Comparison Versus Gold Standard
((15)O-)H2O-Positron Emission Tomography. Stroke. 2018;49(3):621-629. doi:10.1161/STROKEAHA.117.020010.
3. Fan AP,
Khalighi MM, Guo J, et al. Identifying
Hypoperfusion in Moyamoya Disease With Arterial Spin Labeling and an [ 15
O]-Water Positron Emission Tomography/Magnetic Resonance Imaging Normative
Database. Stroke. 2019;50(2):373-380. doi:10.1161/STROKEAHA.118.023426.
4. Juttukonda MR,
Donahue MJ. Neuroimaging of vascular reserve in patients with cerebrovascular
diseases. Neuroimage. 2019;187(August 2017):192-208.
doi:10.1016/j.neuroimage.2017.10.015.
5. Donahue MJ,
Achten E, Cogswell PM, et al. Consensus statement on current and emerging methods
for the diagnosis and evaluation of cerebrovascular disease. J Cereb Blood
Flow Metab. 2018;38(9):1391-1417. doi:10.1177/0271678X17721830.
6. Siero JCW,
Hartkamp NS, Donahue MJ, et al. Neuronal activation induced BOLD and CBF
responses upon acetazolamide administration in patients with steno-occlusive
artery disease. NeuroImage Clin. 2015;105:276-285.
doi:doi:10.1016/j.neuroimage.2014.09.033.
7. Fan AP, Guo J,
Khalighi MM, et al. Long-Delay Arterial Spin Labeling Provides More Accurate
Cerebral Blood Flow Measurements in Moyamoya Patients: A Simultaneous Positron
Emission Tomography/MRI Study. Stroke. 2017;48(9):2441-2449.
doi:10.1161/STROKEAHA.117.017773.
8. Faraco, Carlos
C Strother, Megan Donahue M. Dual echo vessel‐encoded ASL for simultaneous BOLD
and CBF reactivity assessment in patients with ischemic cerebrovascular
disease.pdf. 2014. doi:https://doi.org/10.1002/mrm.25268.
9. Lee M,
Zaharchuk G, Guzman R, Achrol A, Bell-Stephens T, Steinberg GK. Quantitative
hemodynamic studies in moyamoya disease: a review. Neurosurg Focus.
2009;26(4):E5. doi:10.3171/2009.1.FOCUS08300.
10. Hauser T-K,
Seeger A, Bender B, et al. Hypercapnic BOLD MRI
compared to H2(15)O PET/CT for the hemodynamic evaluation of patients with
Moyamoya Disease. NeuroImage Clin. 2019;22:101713.
doi:10.1016/j.nicl.2019.101713.
11. Pellaton A,
Bijlenga P, Bouchez L, et al. CO 2 BOLD assessment of moyamoya syndrome:
Validation with single photon emission computed tomography and positron
emission tomography imaging . World J Radiol. 2016;8(11):887.
doi:10.4329/wjr.v8.i11.887.
12. Goetti R,
Warnock G, Kuhn FP, et al. Quantitative cerebral perfusion imaging in children
and young adults with Moyamoya disease: Comparison of arterial
spin-labeling-MRI and H2[15O]-PET. Am J Neuroradiol.
2014;35(5):1022-1028. doi:10.3174/ajnr.A3799.
13. Sobczyk O,
Battisti-Charbonney A, Fierstra J, et al. A conceptual model for CO2-induced
redistribution of cerebral blood flow with experimental confirmation using BOLD
MRI. Neuroimage. 2014;92:56-68. doi:10.1016/j.neuroimage.2014.01.051.
14. Bhogal AA,
Philippens MEP, Siero JCW, et al. Examining the regional and cerebral
depth-dependent BOLD cerebrovascular reactivity response at 7T. Neuroimage.
2015;114:239-248. doi:10.1016/j.neuroimage.2015.04.014.
Figures
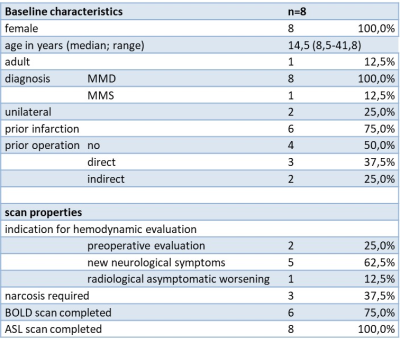
Table 1: Baseline characteristics and scan properties.
Abbreviations: MMD: moyamoya disease; MMS: moyamoya syndrome; BOLD: blood oxygen level dependent; ASL: arterial spin labeling.
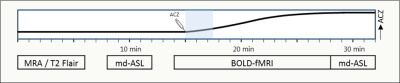
Figure 1: Outline of scan protocol
Preliminary and pragmatic clinical protocol for hemodynamic evaluation. Abbreviations: md-ASL: multidelay arterial spin labeling MRI; BOLD: blood oxygen level dependent MRI. ACZ: acetazolamide. X-axis: time in minutes, with different scan sequences. Y-axis: effect of ACZ on CBF.
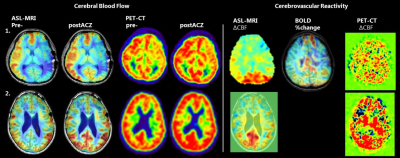
Figure
2: example of 2 patients
Patient 1 is
a 14 y/o girl with unilateral moyamoya for which she received a direct bypass on the left side. She
had new neurological deficits and the scans show a severe perfusion defect on the left,
with still some reactivity. Patient 2 is a 10 y/o girl who received a bilateral
direct bypass. 1 year later the scans show improved CVR on both ASL and PET.
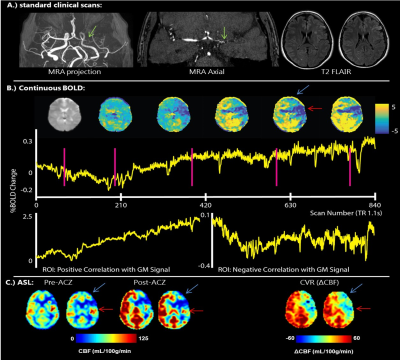
Figure
3: Example of a dataset of a 41 y/o preoperative
patient with unilateral MMV. A.) shows standard clinical scans, MRA and T2
Flair. The green arrow shows
stenosis and formation of collaterals. The BOLD (B) shows the CBF change after
ACZ injection, with impaired CVR left parieto-frontally, also seen on the ASL
(C). There is steal visible (blue arrow)
and arterial transit artifacts (red
arrow). Using the BOLD it is possible to show these apparent high CBF
regions are indeed artefacts and actually show regions with low cerebrovascular reactivity.
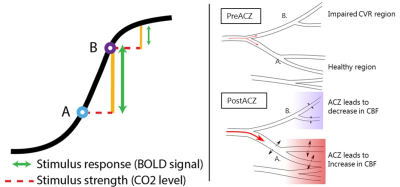
Figure
4: effect of stimulus on different vessels. A is
the (schematic) resting point of healthy vessels and B the resting point of impaired
vessels. The y-axis shows the dilatory capacity, the x-axes the pCO2.
Acetazolamide (ACZ) raises the pCO2. With the same stimulus (red line) different responses are
elicited from both vessels (green line).
Although the impaired vessels may react slightly, the pressure difference after
dilation will lead to redistribution of blood, causing vascular steal in the impaired region (as shown on the right).