1653
Variation in blood-flow pulsatility and arterial distensibility along the internal carotid artery
Rick J van Tuijl1, Ynte M Ruigrok2, Birgitta K Velthuis1, Irene C van der Schaaf1, Gabriël J. E. Rinkel2, and Jaco J.M. Zwanenburg1
1Radiology, UMC Utrecht, Utrecht, Netherlands, 2Neurology and Neurosurgery, Rudolf Magnus Institute of Neuroscience, UMC Utrecht, Utrecht, Netherlands
1Radiology, UMC Utrecht, Utrecht, Netherlands, 2Neurology and Neurosurgery, Rudolf Magnus Institute of Neuroscience, UMC Utrecht, Utrecht, Netherlands
Synopsis
Velocity pulsatility and area distensibility variation along the internal carotid artery (ICA) trajectory were studied with 2D phase-contrast flow measurements (118 subjects; 3T MRI). Results showed a more complicated behavior than just progressive pulsatility decrease along the ICA towards the circle of Willis. The bony carotid canal appears to constrain the distensibility of the ICA, yielding locally increased velocity pulsatility. Velocity pulsatility was significantly higher in men compared to women and increased with ageing. This age-related increase was strongest just proximal to the circle of Willis, indicating reduced damping of the pulsatility along the ICA with age.
Introduction
Decrease of the blood flow pulsatility along the internal carotid artery (ICA) is deemed necessary to protect the microvasculature of the brain. Increased pulsatility is associated with cardiovascular disease, stroke and vascular dementia (1) and probably with the development of intracranial aneurysms (2). The role of the carotid siphon within the whole ICA trajectory in damping the pulsatility is still poorly understood. This study aims to 1) assess velocity pulsatility and arterial distensibility of the ICA with velocity phase contrast MRI measurements at three segments, covering both extracranial and intracranial parts, and 2) test the influence of age and sex on pulsatility and distensibility.Methods
Data were obtained from the ongoing study “Early Recognition of persons at high risk of Aneurysmal Subarachnoid hemorrhagE (ERASE)”. Patients underwent 3T MRI (Philips; 32-channel head-neck coil) including 2D phase contrast velocity measurements at three segments of the ICA: extracranial (C1), carotid canal (C3), and intracranial (C7), see Figure 1. 2D phase contrast parameters: resolution 0.5x0.5x3mm3, Venc: 90/90/150cm/s (C1/C3/C7), FOV: 250x250mm2. Semi-automated analysis of the flow measurements was performed using the software of the scanner console. Contours were automatically created and propagated for the entire cardiac cycle after a single mouse click in the respective vessel (right ICA, left ICA and Basilar artery (BA) for every segment. The BA served as reference for variation in pulsatility and distensibility along a straight vessel segment. Analysis results of a subject were included if the following criteria were met for all segments of at least one vessel: Image slice perpendicular to the vessel: the contour is circular and regular for all three segments over all heart phases. Correct segmentation: the contour follows the vessel lumen for every segment with only minor variation between heart phases (limited effect of noise). From the results, the velocity pulsatility index (vPI) was calculated as vPI = (Vmax-Vmin)/Vmean) with V the time curve of the region-of-interest’s mean velocity. The area pulsatility index (aPI, as metric for vessel wall distensibility) was calculated as aPI = (Amax-Amin)/Amean with A the area of the vessel lumen. To compare vPI and aPI, paired t-tests were used. The association of age and sex with vPI and aPI was assessed with a linear mixed-effects model with segment as mixed-effect for vPI and aPI. The significance threshold was p<0.05.Results
Table 1 shows the baseline characteristics of the 118 participants. vPI increased between segments C1 and C3 (0.85±0.13 vs. 0.93±0.13, p<0.0001 for averaged right+left ICA) and decreased over the carotid siphon between segments C3 and C7 (0.93±0.13 vs. 0.84±0.13, p<0.0001) (Table 2/Figure 2). No vPI difference was found over the whole ICA (C1-C7). Conversely, the aPI (distensibility) decreased between C1 and C3 (0.18±0.06 vs 0.14±0.04, p<0.0001) and increased between C3 and C7 (0.14±0.04 vs 0.31±0.09, p<0.0001). The observations were consistent for both ICAs and between individuals: The trend over C1-C3-C7 as reported in the average values was seen in 168 out of 171 individual vPI ICA measurements (98%) and in 162 out of 171 aPI measurements (95%). vPI in men is higher than in women at all segments: C1 (0.92±0.13 vs 0.79±0.09, p<0.0001), C3 (1.01±0.13 vs 0.87±0.09, p<0.0001) and C7 (0.92±0.14 vs 0.79±0.09, p<0.0001). vPI increased with age (p<0.015) with highest age-related increase in vPI at the C7 level (vPI=0.677+0.0036∙age(years)). The decrease of the vPI over the carotid siphon declined with age (vPIC3/vPIC7=1.167–0.0009∙age(years)).Discussion
We found an increase in the vPI between the extracranial C1 segment and the C3 segment (carotid canal) of the ICA, and a subsequent decrease over the carotid siphon (from C3 to C7). At C3, the ICA wall is constrained by the rigid boundaries of the bony carotid canal, which probably explains the significantly decreased distensibility (aPI) and increased vPI, relative to those values at the other two levels, where the ICA is surrounded by soft tissue (C1), or cerebral spinal fluid (C7), respectively. Although we confirmed previous studies showing attenuation of vPI over the carotid siphon (C3-C7 level) (4, 5), our results suggest that the decrease over the siphon may be interpreted as normalization of the pulsatility rather than an effective decrease. The lack of vPI variation over segments of the straight BA confirms the observed vPI damping function of the siphon. Our study showed that the damping of vPI over the carotid siphon decreased at increasing age. This probably reflects progressive stiffening of the carotid siphon with increasing age (6) and is in line with literature (7, 8).Conclusion
The pulsatility along the ICA shows a more complicated behavior than just progressive pulsatility decrease towards the circle of Willis. The bony carotid canal seems to constrain the distensibility of the ICA, thus locally increasing the velocity pulsatility at carotid canal segment C3. This may render the damping function of the carotid siphon (between C3 and the circle of Willis) extra relevant. Future studies should focus on the influence hypertension and vessel wall calcification on pulsatility and distensibility of the ICA.Acknowledgements
We acknowledge the support of the Netherlands CardioVascular Research Initiative: the Dutch Heart Foundation (CVON 2015-008 ERASE), Dutch Federation of University Medical Centers, the Netherlands Organization for Health Research and Development, and the Royal Netherlands Academy of Sciences.References
1. Jagtap A, Gawande S, Sharma S. Biomarkers in vascular dementia: A recent update. Biomarkers and Genomic Medicine. 2015;7:43-56.2. Chang CW, Wai YY, Lim SN, Wu T. Association Between Flow Acceleration in the Carotid Artery and Intracranial Aneurysms. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2018.
3. Bouthillier A, van Loveren HR, Keller JT. Segments of the internal carotid artery: a new classification. Neurosurgery. 1996;38:425-32.
4. Schubert T, Santini F, Stalder AF, Bock J, Meckel S, Bonati L, et al. Dampening of Blood-Flow Pulsatility along the Carotid Siphon: Does Form Follow Function? American Journal of Neuroradiology. 2011;32:1107-12.
5. Zarrinkoob L, Ambarki K, Wahlin A, Birgander R, Carlberg B, Eklund A, et al. Aging alters the dampening of pulsatile blood flow in cerebral arteries. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2016;36:1519-27. 6. McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2006;113:30-7.
7. Tarumi T, Khan MA, Liu J, Tseng BM, Parker R, Riley J, et al. Cerebral Hemodynamics in Normal Aging: Central Artery Stiffness, Wave Reflection, and Pressure Pulsatility. Journal of Cerebral Blood Flow & Metabolism. 2014;34:971-8.
8. Xing C-Y, Tarumi T, Liu J, Zhang Y, Turner M, Riley J, et al. Distribution of cardiac output to the brain across the adult lifespan. Journal of Cerebral Blood Flow & Metabolism. 2017;37:2848-56.
Figures
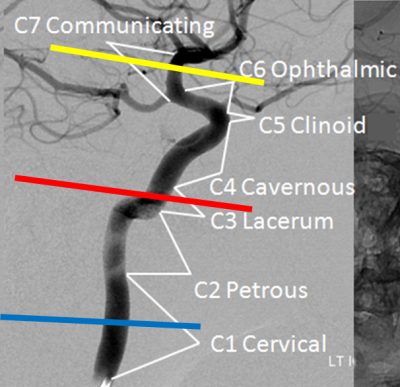
Figure 1: Illustration of the planning of the 2D phase
contrast velocity measurements at the different segments C1 (blue line), C3
(red) and C7 (yellow) of the ICA. Also, the segments according to the
classification of Bouthillier (3) are indicated.
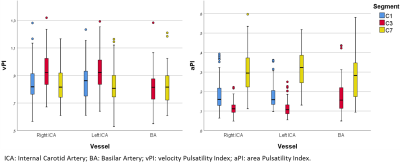
Figure 2: Velocity pulsatility index (vPI, left) and area pulsatility index (aPI, right) per vessel for each
segment, showing inverse trends across segments: The area pulsatility (vessel
wall distensibility) decreases between C1 and C3, and subsequently increases
again to C7, the velocity pulsatility increases between C1 and C3 and decreases
again at C7.
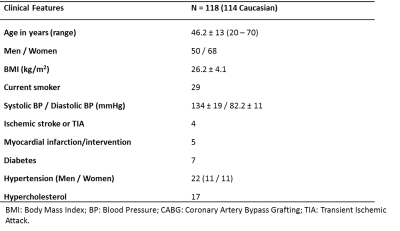
Table 1: Baseline characteristics of the 118 subjects that
were included in this study.
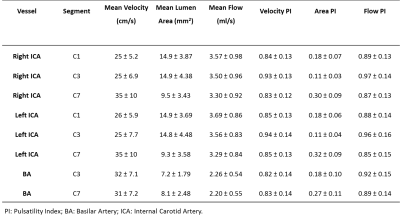
Table 2: Schematic overview of hemodynamic parameters
and velocity pulsatility index, area pulsatility index and flow pulsatility
index for every vessel per segment. (As flow = velocity*area, we focused separately on
the velocity- and area pulsatility.)