1648
Deep learning based Ischemic core prediction from perfusion-weighted imaging in acute ischemic stroke
Yannan Yu1, Yuan Xie1, Thoralf Thamm1, Enhao Gong1, Jiahong Ouyang1, Soren Christensen1, Michael P Marks1, Maarten G Lansberg1, Gregory W Albers1, and Greg Zaharchuk1
1Stanford University, Stanford, CA, United States
1Stanford University, Stanford, CA, United States
Synopsis
Ischemic core of acute ischemic stroke is commonly defined by diffusion-weighted imaging (DWI). CT perfusion, although widely used for acute stroke triaging, is challenging to identify the ischemic core as precise as DWI. In this study, we predicted the DWI lesion from MR perfusion-weighted imaging using U-Net. We found U-net model can predict the ischemic core from perfusion imaging with a better performance compared to clinically-used relative cerebral blood flow map thresholding. In the future study, we will apply the model to patients underwent CT perfusion using transfer learning.
Background and Objective
Ischemic core is commonly defined by diffusion-weighted imaging (DWI). On CT perfusion, although widely used for acute stroke triaging, it is challenging to identify the ischemic core as precisely as DWI. In this study, we aim to predict the DWI lesion from MR perfusion-weighted imaging alone using a U-Net deep learning architecture, with the ultimate goal of applying this to CT perfusion studies.Methods
Acute ischemic stroke patients from DEFUSE2 (NCT01349946) and iCAS (NCT02225730) trials were included if they had baseline DWI and PWI. RAPID software (iSchemaview, Redwood City, CA) was used to reconstruct perfusion parameter maps: Tmax, mean transit time (MTT), cerebral blood volume (CBV), and cerebral blood flow (CBF). This software also automatically generates an ischemic core (ADC segmentation) with a threshold of < 620 × 10-6 mm2/s. An attention-gated U-net was trained with six image channels from baseline perfusion maps (Tmax, MTT, CBF, CBV, segmentation of Tmax > 6s, and rCBF ≤ 30%) as inputs and the RAPID ischemic core as ground truth. Five-fold cross-validation was performed. Models were evaluated using Dice score coefficient (DSC) and lesion volume difference and compared with the current “CTP-derived” thresholding method (rCBF ≤ 30% within the area of hypoperfusion). Acute ischemic stroke patients from DEFUSE 2(NCT01349946) and ICAS (NCT02225730) trials were included if they had baseline DWI and PWI. RAPID software was used to reconstruct perfusion parameter maps: Tmax, mean transit time (MTT), cerebral blood volume (CBV), and cerebral blood flow (CBF). This software also automatically generates ADC segmentation with a threshold of < 620 × 10-6 mm2/s. An attention-gated U-net was trained with six image channels from baseline perfusion maps (Tmax, MTT, CBF, CBV, segmentation of Tmax > 6s and rCBF ≤ 30%) as inputs and the ADC segmentation from RAPID as ground truth. Five-fold cross-validation was performed. Models were evaluated using Dice score coefficient (DSC), and lesion volume difference at a cutoff of 0.5, and compared with the current thresholding method (rCBF ≤ 30% within the area of hypoperfusion).Results
182 patients were included (85 males, age 65±16 yrs). In 154 patients with a DWI lesion, the U-net model performed better than the thresholding method (Fig A), with a median DSC of 0.52 (interquartile range [IQR] 0.30, 0.64) vs 0.38 (IQR 0.24, 0.48, p<0.001), volume difference of -3 ml (IQR -17, 10) vs -20 ml (IQR -42, -7, p<0.001), and absolute volume difference 15 ml (6, 31) vs 21 ml (IQR 10, 43, p<0.001). In 28 patients without a DWI lesion, the U-net had a median volume difference of 6 ml (IQR 1, 15)(Fig B).Conclusions
A U-net model can predict the ischemic core from perfusion imaging with a better performance compared to clinically used thresholding. In the future study, we will apply the model to patients undergoing CT perfusion using transfer learning.U-net model can predict the ischemic core from perfusion imaging with a better performance compared to clinically used thresholding. In the future study, we will apply the model to patients underwent CT perfusion using transfer learning.Acknowledgements
No acknowledgement found.References
No reference found.Figures
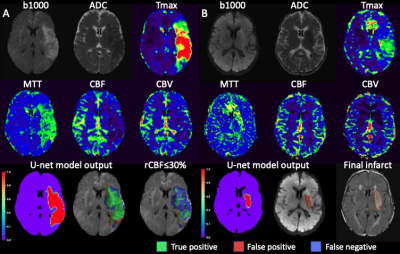
Fig A. A case with excellent DWI
lesion prediction from the U-net model. RAPID measured a DWI lesion volume of 173
ml. The U-net model achieved a DSC of 0.72 and volume of 149 ml, while the rCBF
thresholding had a DSC of 0.46 and volume of 58 ml. Fig B. A case with positive
DWI but no RAPID ischemic core lesion. The patient developed basal ganglia
infarction despite 100% reperfusion within 24 hours. The final infarct on
follow-up FLAIR and the U-net model prediction both had a volume of 7 ml.