1642
Differential Correlations Between White Matter Microstructure and Perfusion Reveal Microvascular Dysregulation in Sickle Cell Anemia
Soyoung Choi1,2, Chau Vu3, Richard M Leahy4, and John Wood5
1Neuroscience Graduate Program, University of Southern California, LOS ANGELES, CA, United States, 2Children's Hospital Los Angeles, LOS ANGELES, CA, United States, 3Biomedical Engineering, University of Southern California, LOS ANGELES, CA, United States, 4Signal and Image Processing Institute, University of Southern California, Los Angeles, CA, United States, 5Division of Hematology, Children's Hospital Los Angeles, Los Angeles, CA, United States
1Neuroscience Graduate Program, University of Southern California, LOS ANGELES, CA, United States, 2Children's Hospital Los Angeles, LOS ANGELES, CA, United States, 3Biomedical Engineering, University of Southern California, LOS ANGELES, CA, United States, 4Signal and Image Processing Institute, University of Southern California, Los Angeles, CA, United States, 5Division of Hematology, Children's Hospital Los Angeles, Los Angeles, CA, United States
Synopsis
Patients with chronic anemia has been shown to be susceptible to structural, functional and cognitive impairments. This study explores differences in white matter microstructure (measured by ADC) and cerebrovascular perfusion patterns (measured by MTT) and their relationship to hemoglobin in patients with sickle cell anemia, non-sickle anemia and controls. Larger ADC values and faster MTT is found along the anterior circulation of the brain in sickle-cell anemic patients. Relationships were found between hemoglobin, ADC and MTT in controls that were not observed in sickle-cell patients possibly indicating microvascular dysregulation. Our observations indicate possible microvascular dysregulation in sickle cell anemic patients.
Introduction
Patients with chronic anemia has been reported to show structural, functional and cognitive impairments in the brain. The brain is autoregulated to respond to low hemoglobin and oxygen through compensatory cerebral vasodilation. Although a global rise in cerebral blood flow has been shown in anemic patients,[1] it has been proposed that microvascular dysregulation may still be adversely affecting these patients.[2] Our laboratory previously characterized lower white matter brain volume in sickle and non-sickle anemic patients associated with low cognitive scores.[3], [4] and found that oxygen delivery in white matter was 35% lower in anemic patients in comparison to controls.[5] Based on these observations, we hypothesize that loss of integrity in white matter tissue could be related to disrupted hyperemic response.Here we investigate whether differences in white matter tissue microstructure can be detected in our anemic patients. Diffusion weighted imaging allows us to infer information about microstructure as we can measure the restricted diffusion of water in white matter tissue. We used apparent diffusion coefficient (ADC) to measure white matter integrity as increased values of ADC has been associated with compromised brain tissue.
We then explore how tissue microstructure relates to the brain’s perfusion patterns using deoxygenation-based DSC. Red blood cells deliver oxygen to tissue along capillaries. Mean transit time (MTT) measures the amount of time that blood dwells at the capillaries, allowing sufficient oxygen exchange with the surrounding tissue. We hypothesize that insufficient mean transit time would explain local disruptions to tissue microstructure. We tested our hypothesis in sickle cell anemic (SCA) patients, non-sickle anemic (NSA) patients and controls (CTL).
Methods
MRI data and complete blood panels were obtained from SCA (age=20.7±8.1, F=17, M=17, HgB = 9.8±1.8), NSA (age=25.3±9.5, F=8, M=4, HgB=10.7±2.0) and CTL subjects (age=26.4±10.8, F=27,M=7, HgB=13.3±1.2). (Recruited with informed consent or assent; IRB: CHLA CCI#11-00083; 3T Philips Achieva v.3.2.1., 8-channel head coil). 3D T1-weighted images (TE =3.8ms TR =8.3ms; resolution = 1mm3) were pre-processed using BrainSuite (brainsuite.org, v18a) and labeled using the USCLobes atlas. DWI (TE = 2.5ms; TR = 4.8ms; resolution=2.5mm3) included 30 diffusion-encoding directions at b-value=1000m/s2 and a reverse-gradient b=0. Fieldmaps were estimated using FSL’s topup module. BrainSuite Diffusion Pipeline (BDP18a) performed distortion correction, co-registration to T1-weighted images and calculated ADC maps. Only ADC values in white matter tissue were retained.BOLD images (TE/TR=50ms/2000ms; flip angle=90°; resolution=2.3x2.3x5mm) were acquired while subjects were fitted with a rebreathing apparatus where 5 inhalations of 100% nitrogen gas administered then replaced by room air gas mixture. FSL was used for motion correction, registration to MNI template (labelled by USCLobes atlas) and smoothing (8mm3 gaussian kernel). Arterial input function was extracted from the middle cerebral artery. Regional cerebral blood volume and blood flow was computed from adapted DSC equations [6]. Mean transit time was calculated as the ratio between blood volume and blood flow based on central volume theorem.[7] Mean ADC and MTT were computed in each ROI of the USCLobes atlas then controlled for age and sex. SCA, NSA and CTL subjects were compared using student’s t-tests. Pearson’s correlations were run between ADC and MTT in each ROI by group.
Results
Sickle cell anemic patients had larger ADC values bilaterally in the frontal and temporal lobes, right parietal lobe, brainstem and corpus callosum in comparison to controls. MTT was lower in SCA patient compared to controls in the bilateral frontal and parietal lobes and the left temporal lobe. (Table 1) Hemoglobin was negatively correlated to ADC in the brainstem and cerebellum of NSA subjects but positively correlated in the brainstem of control subjects. Lower hemoglobin levels were associated with faster MTT bilaterally in the parietal and occipital lobes in controls only. SCA patients only showed a positive correlation between hemoglobin and MTT in deep white matter. (Table 2) ADC and MTT were positively correlated throughout the brain in control subjects while no correlations were observed in anemic patients. (Table 2)Discussion
Evidence of compromised white matter microstructure and short perfusion time was found in SCA along anterior cerebral circulation territories. Lower red blood cell dwelling time in capillaries may explain lower oxygen delivery in white matter tissue observed previously.[5]While hemoglobin alone could not predict ADC values, longer MTT was associated with higher hemoglobin and larger ADC in control subjects. This may be indicative of the intricacies of the autoregulatory system in response to both oxygen supply and demand. Short MTT in response to low hemoglobin may indicate increased cerebral blood flow to maintain normal oxygen delivery to the brain while long MTT in response to high ADC may indicate cerebral vasodilation in response to higher demand of oxygen in compromised tissue. Regardless of hemoglobin level or ADC, we did not observe significant difference in MTT, possibly indicating microvascular dysregulation.
Significant differences in ADC and MTT were not observed in NSA subjects possibly due to low sample size. The deoxygenation-based DSC used in this report was utilized to mirror dynamic susceptibility contrast (DSC) imaging that requires the use of a contrast agent. While we assume that brief duration of desaturation does not cause significant change in global flow, we recognize that acute hypoxia may have confounded our MTT measures.
Acknowledgements
This work was supported by National Heart, Lung, and Blood Institute (1U01-HL-117718-01, 1RO1HL136484-A1 and a Minority Supplement to 1U01-HL-117718-01), the National Center for Research (5UL1TR000130-05) through the Clinical Translational Science Institute at Children’s Hospital Los Angeles, the National Institute of Neurological Disorders and Stroke (grant 5R01NS074980-07, 1F31NS106828-01A1, R01NS074980) and the National Institutes of Health Predoctoral Training in Interdisciplinary Neurosciences (1T32MH111360-1A1). Philips Healthcare provided support for protocol development and applications engineering on a support-in-kind basis.References
- M. T. Borzage et al., “Predictors of cerebral blood flow in patients with and without anemia.,” J. Appl. Physiol., vol. 120, no. 8, pp. 976–81, Apr. 2016.
- J. A. Detterich et al., “Sickle cell microvascular paradox—oxygen supply-demand mismatch,” Am. J. Hematol., no. March, pp. 678–688, 2019.
- S. Choi et al., “Hemoglobin and mean platelet volume predicts diffuse T1-MRI white matter volume decrease in sickle cell disease patients,” NeuroImage Clin., vol. 15, no. January, pp. 239–246, 2017.
- S. Choi et al., “Anemia predicts lower white matter volume and cognitive performance in sickle and non‐sickle cell anemia syndrome,” Am. J. Hematol., p. ajh.25570, Jul. 2019.
- Y. Chai et al., “White Matter Has Impaired Resting Oxygen Delivery in Sickle Cell Patients,” Am. J. Hematol., no. December 2018, pp. 1–8, 2019.
- C. Vu, J. Coloigner, S. Choi, and J. Wood, “Relative perfusion mapping using BOLD imaging with induced hypoxia,” in International Society of Magnetic Resonance in Medicine Annual Meeting, 2018, p. 2292.
- K. L. Zierler, “Theoretical Basis of Indicator-Dilution Methods For Measuring Flow and Volume,” Circ. Res., vol. 10, no. 3, pp. 393–407, Mar. 1962.
Figures
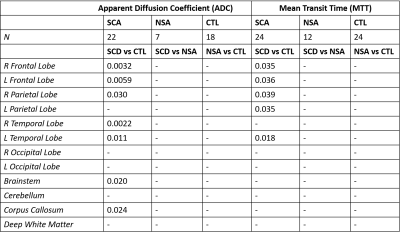
Table 1. Group differences
between sickle cell anemic (SCA), non-sickle anemic (NSA) and control (CTL)
subjects in ADC (left) and MTT (right). pvalues of significant group
differences observed are shown (p≤0.05). R: right; L: left.
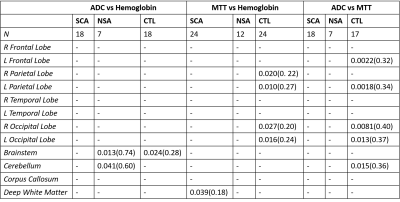
Table 2. Pearson’s
correlationships performed on apparent diffusion coefficient (ADC) vs
hemoglobin (left), mean transit time (MTT) vs hemoglobin (middle) and ADC vs
MTT (right). Tests were performed separately by disease state: sickle cell
anemic (SCA), non-sickle anemic (NSA) and control (CTL) subjects. pvalues(r2)
is displayed where significant correlations were observed (p≤0.05). R: right; L: left.