1640
Hemodynamic changes underpinning the discrepancy of task performance in HIV+ patients
Danhui Fu1, Guanqiao Jin1, Wenjuan Deng1, Weiyin Vivian Liu2, Long Qian2, and Danke Su1
1GuangXi Medical University Cancer Hospital, Nanning, China, 2MR Research, GE Healthcare, Beijin, China
1GuangXi Medical University Cancer Hospital, Nanning, China, 2MR Research, GE Healthcare, Beijin, China
Synopsis
A bulk of research shows reduced cerebral blood flow is linked to thinning of the cortex and cognitive impairment in dementias and vascular disease while negative relations between functional connectivity and cognition in health are positive in HIV. Our findings of significantly increased CBF in the bilateral middle occipital gyri (MCG) while only left MCG negatively correlated with time for Stroop Color in HIV+ group might underpin that the transition from normal cognition to cognitive impairment in compensation for the abnormal structural and functional alterations. CBF values might reflect hemodynamic differences in HIV+ patients without signs of HIV-associated neurocognitive disorder.
Introduction
Structural imaging studies have indicated there is a link between level of cerebral atrophy, white matter hyperintensities (WHM) and function impairment while functional imaging research has shown that impaired cognition was associated with lower brain activities in the regional brain, especially in the frontoparietal network (FPN), default mode network (DMN) and sensorimotor network (SMN). 1-7A bulk of research shows reduced cerebral blood flow (CBF) is linked to thinning of the cortex and cognitive impairment in neurodegenerative dementias and vascular disease. 2-5 However, decreased regional CBF (rCBF) can coexist with increased rCBF in the early stages of the neurodegenerative process. For example, rCBF is elevated in the medial temporal lobe, amygdala, and anterior cingulate gyrus in patients with mild cognitive impairment (MCI), suggesting a compensatory increase in neural activity. 6 Moreover, a recent report indicates that HIV infection leads not only to weakened but also strengthened resting-state functional connectivity (FC). Negative relations between FC and cognition in health are positive in HIV. 1 Therefore, this study aims to explore how cerebral blood flow dominates in HIV-positive (HIV+) patients without HIV-associated neurocognitive disorder (HAND) compared to healthy volunteers.Materials and Methods
This study was approved by the Institutional Review Board of Guang-Xi Medical University Cancer Hospital and all recruited individuals gave the informed consent. Patients in our registry database from September 2017 to January 2019 were reviewed and patients age between 19 and 63 with complete data sets and without receiving combination antiretroviral therapy (CART) were included in the study. Subjects with matched age, gender, education and MMSE were enrolled as healthy controls (HCs). All participants underwent neuropsychological tests (Mini-Mental State Examination [MMSE], Digit symbol, color trails, digital span test, verbal fluency, Stroop test [Stroop C and Stroop CW] ). Routine axial T1-weighted structural BRAVO imaging and arterial spin labeling (ASL) imaging were performed on 3.0T clinic scanner (Discovery 750w, GE Healthcare, USA). The CBF images were obtained from the work station manufactured by GE Healthcare. To extract the atlas based CBF values, T1 anatomical images of each subject were first co-registered to CBF images. Then, the co-registered T1 images were normalized to MNI space using SPM12. Thereafter, all the CBF images could be transformed to individual MNI space. Last, all normalized CBF images were used to extract the CBF values based on 90 AAL atlas-based brain regions with custom-designed code. SPSS 19.0 was used to assess the discrepancy and correlation of CBF and neuropsychological tests between two groups.Results and Discussion
Twenty-two HIV+ patients in the experimental group and seventeen healthy volunteers in the control group were confirmed well-balanced with equal variances by independent sample t-test. The CBF in right precentral and postcentral gyri, right calcarine fissure and surrounding cortex, right cuneus, bilateral lingual gyri, bilateral middle occipital gyri and right superior parietal gyrus (p< 0.05) along with time for Stroop Color (SC) (p= 0.005) were significantly higher in HIV-positive group than in HCs (shown in Table 1). Moreover, in HIV+ group, left occipital gyrus was statistically negatively correlated with SC time (r= -0.457, p= 0.038) while no significant correlation was found in HCs (shown in Figure 1). Our findings of increases in blood flow linked to decreases in brain function disobeyed the known evidences that decreased CBF is linked to thinning of the cortex and cognitive impairment in neurodegenerative dementias and vascular disease 1-4 while supported that HIV may lead to thickening of the left occipital lobe but thinning cortical thickness of the right temporal lobe and higher ReHo in bilateral superior parietal gyrus (SPG)/superior occipital gyrus (SOG) in the white matter hyperintensity (WMH) with CI group than without CI group. 1 Moreover, mild cognitive impairment (MCI) is expressed as both decreases and increases in rCBF. 7 Hence, we might explain increased CBF, especially bilateral middle occipital gyri, was a compensatory mechanism to maintain the regional brain to perform normal as the transition stage from normal cognition to CI in the neurodegenerative process. In our results, all aforementioned regions except for right precentral gyrus and left lingual were significantly positive correlated with each other (p<0.001) when controlling for age. This further underpinned most regions synergistically responded to Stroop Color tests even HIV+ patients spent more time to react each trial. To sum up, CBF values might reflect hemodynamic differences in HIV+individuals with no obvious signs of MCI and distinguish them from HCs. Further research on discrimination of HIV+patients with and without MCI is need.Conclusions:
Non-invasive ASL imaging without an external tracer may be an early discriminator for HIV+ or HIV- patients ahead of functional and structural alterations.Acknowledgements
No acknowledgement found.References
- Ye Q, Chen X, Qin R, Huang L, et. al (2019) Enhanced Regional Homogeneity and Functional Connectivity in Subjects With White Matter Hyperintensities and Cognitive Impairment. Front. Neurosci. 2019; 13: 695
- Weston PS, Nicholas JM, Lehmann M, et al. Presymptomatic cortical thinning in familial Alzheimer disease: A longitudinal MRI study. Neurology. 2016; 87(19):2050–7.
- Krumm S, Kivisaari SL, Probst A, et al. Cortical thinning of parahip- pocampal subregions in very early Alzheimer’s disease. Neurobiol Aging. 2016; 38:188–96.
- Kim HJ, Ye BS, Yoon CW, et al. Cortical thickness and hippocampal shape in pure vascular mild cognitive impairment and dementia of subcortical type. Eur J Neurol. 2014; 21 (5):744–51.
- Seo SW, Ahn J, Yoon U, Im K, Lee JM, Tae Kim S, et al. Cortical thinning in vascular mild cognitive impairment and vascular dementia of subcortical type. J Neuroimaging. 2010; 20(1):37–45.
- Alsop DC, Casement M, de Bazelaire C, et al. Hippocampal hyperperfusion in Alzheimer’s disease. Neuroimage.2008;42:1267–1274.
- Dai WY, Lopez OL, Carmichael OT, et al. Mild Cognitive Impairment and Alzheimer Disease: Patterns of Altered Cerebral Blood Flow at MR Imaging. Radiology. 2009; 250: 856-866
Figures
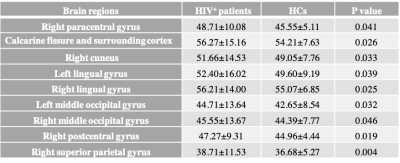
Table 1 Only significant different CBF values (in unit of ml/100g/min) between HIV+ patients and HCs in the overall 90 AAL-atlas based brain regions are listed.
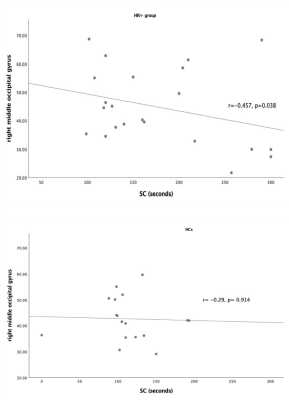
Figure 1 The correlation between right middle occipital gyrus and time for Stroop Color tests in HIV+ patients and HCs. Significant correlation was only found in the experimental group.