1637
Anemia, silent stroke, and vascular abnormalities independently contribute to microstructural integrity in Tanzanian sickle cell patients1Imaging and Biophysics, Developmental Nerosciences, UCL Great Ormond St Institute of Child Health, London, United Kingdom, 2Department of Radiology & Imaging, Muhimbili University of Health and Allied Sciences, Dar Es Salaam, Tanzania, 3Department of Radiology, Great Ormond Street Hospital, London, United Kingdom
Synopsis
Reductions in white matter integrity are associated with neurocognitive dysfunction in sickle cell anemia (SCA) patients, but the aetiology is poorly understood. Aiming to explore whether anemia severity, silent cerebral infarction (SCI), and vascular abnormalities may all play a role, we conducted tract-based-spatial statistics in 62 Tanzanian children with SCA. We found anemia severity to be independently associated with increased mean and axial diffusivity, SCI with increased radial diffusivity, and turbulence or vasculopathy with reduced fractional anisotropy. Our findings are consistent with a model of neurological complications in which these pathologies may all contribute to functionally-significant reductions in tissue integrity.
Introduction
Patients with sickle cell anemia (SCA) are at risk of neurological complications, including overt and silent stroke (SCI), both of which contribute to cognitive dysfunction1. Stroke and SCI are often, but not always, associated with underlying large-vessel vasculopathy2. In recent years, evidence has emerged indicating that reduced white matter integrity, quantifiable using diffusion tensor imaging (DTI), may be more common3, and potentially also more functionally significant, e.g. in terms of cognitive disability, than SCI alone in SCA patients4.As there are limited DTI data from SCA cohorts worldwide, reductions in white-matter integrity remain poorly understood. Moreover, despite sub-Saharan Africa bearing the majority of the world’s SCA burden, none of the previous DTI studies were conducted there. Whilst studies in the North have demonstrated relationships between DTI metrics and anemia and oxygen desaturation3, potential independent effects of SCI and vascular abnormalities have yet to be explored. Aiming to establish whether anemia, SCI, and vascular abnormalities are all independently associated with reductions in white-matter integrity, we conducted tract-based spatial statistics (TBSS) in Tanzanian children with SCA.
Methods
MRI Acquisition and EvaluationParticipants were scanned on a 1.5T Phillips Achieva using a 16-channel phased-array head coil. DTI data were acquired using a single-shot echo-planar spin-echo sequence (repetition time=8100ms, echo time=35 ms, slice thickness=2mm, 60 axial slices, 12 noncollinear gradient directions, with b-values=0ms, 1000s/mm2, scan time=8min). The MRI protocol also included standard diagnostic images, with T2-weighted, fluid-attenuated-inversion recovery (FLAIR), and 3-D magnetic-resonance angiography (MRA) sequences. Clinical T2, FLAIR, and MRA images were evaluated by two neuroradiologists (MJ, DS). SCI were diagnosed according to the criteria of a hyperintensity on T2/FLAIR of at least 3mm in greatest diameter and visible on two-planes5. Vascular abnormalities were graded according to the severity of the signal loss on MRA (0 - none; 1 – mild, minor signal attenuation/turbulence; 2 – moderate, obvious signal attenuation with presence of distal flow; 3 – severe, signal loss and no distal flow)6. Anemia severity was measured using hemoglobin concentration, recorded from the closest available full blood count.
MRI Processing
Susceptibility-induced distortions and eddy-currents were corrected using FSL (FMRIB, Oxford, UK). Maps for DTI parameters were generated by fitting a diffusion tensor model to each voxel via a weighted least squares method, providing voxel-wise fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD) parameter maps. As described in previous work7, parameter maps were analysed following a whole-brain voxel-wise TBSS approach. Correlations and group-comparisons were performed with age, sex, and other variables under consideration (SCI, vascular abnormalities, and/or hemoglobin) included in models as covariates. Threshold-free cluster enhancement was used to correct for multiple comparisons.
Results
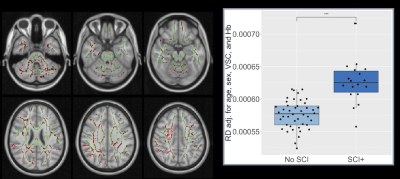
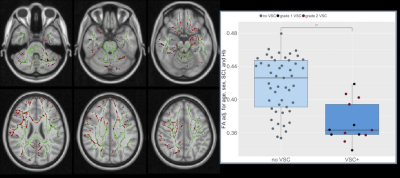
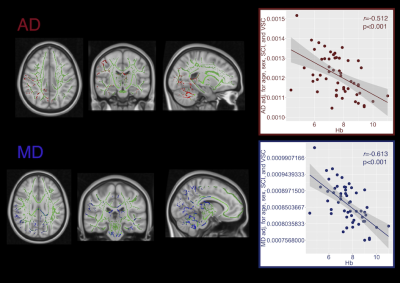
Of 62 patients (mean age=12.97, range=6-19 years, 26 male) with useable DTI data, 17 were identified with SCI, and 13 with vascular abnormalities (6 grade 1 - turbulence, 7 grade 2 - mild-moderate vasculopathy).Patients with SCI exhibited significantly increased RD in widespread regions, whereas those with vascular abnormalities showed significantly decreased FA, primarily in anterior regions (Fig 1. & 2, both p<0.05). Significant negative correlations were also observed between hemoglobin concentration and RD and AD in a number of posterior regions (Fig. 3), including the cingulum, superior longitudinal fasciculus, corpus callosum, internal capsule, corticospinal tract, and cerebellar peduncles (p<0.05).
Discussion
This initial study exploring white-matter integrity in Tanzanian SCA patients provides evidence for independent associations between DTI parameters and anemia, SCI, and turbulence or vasculopathy. Affected regions varied between the studied pathologies. The patterns observed may relate to the underlying pathophysiology.Compensatory steal8 from the posterior circulation may, for example, account for the anemia-associated integrity reductions observed in posterior regions. Prior work has demonstrated that anemia is associated with increased cerebral blood flow (CBF) in SCD patients, which may exhaust cerebrovascular reserves9. There is evidence that in healthy individuals, CBF increases are greater in frontal regions during hypobaric hypoxia10, consistent with the notion that posterior regions may be vulnerable to compensatory steal under conditions of hemodynamic stress.
Moreover, the widespread changes in integrity observed with SCI may indicate downstream effects of lesions on tissue integrity. It is however also possible that subtle alterations in integrity precede the development of lesions, as has been observed in other conditions11.
The anterior integrity changes observed with vascular abnormalities are consistent with those abnormalities involving the anterior and middle cerebral arteries in the majority of patients. Integrity reductions were, however, observed irrespective of whether MRA abnormalities were mild (turbulence) or moderate (vasculopathy), and there appeared to be no relationship between the degree of MRA change and the average degree of integrity reduction (Fig. 2). It is possible that turbulence represents early vascular abnormalities that do not reduce vessel calibre, but nevertheless exacerbate hemodynamic stress. Of note, integrity reductions in patients with MRA abnormalities were also observed in posterior regions, indicating that factors beyond downstream flow disturbances may be involved. Further work is required to establish whether other co-occurring haemodynamic stressors could mediate or moderate the observed associations between microstructural integrity and hemoglobin, SCI, and turbulence or vasculopathy.
Conclusion
These findings are consistent with a model of neurological complications in which anemia, SCI, and vascular abnormalities may all contribute to functionally-significant reductions in tissue integrity.Acknowledgements
We would like to thank Prof. Julie Makani, Dr. Raphael Z. Sangeda, Dr. Clara Chamba, and Dr. Ramadhan Kazema for their contribution to this work, and the participants and their families.
References
1. Prussien K V, DeBaun MR, Yarboi J, et al. Cognitive Function, Coping, and Depressive Symptoms in Children and Adolescents with Sickle Cell Disease. J Pediatr Psychol 2018; 43(5):543–51.
2. Guilliams KP, Fields ME, Ragan DK, et al. Large-Vessel Vasculopathy in Children With Sickle Cell Disease: A Magnetic Resonance Imaging Study of Infarct Topography and Focal Atrophy. Pediatr Neurol 2017; 69:49–57.
3. Kawadler JM, Kirkham FJ, Clayden JD, et al. White Matter Damage Relates to Oxygen Saturation in Children With Sickle Cell Anemia Without Silent Cerebral Infarcts. Stroke 2015; 46(7):1793–9.
4. Stotesbury H, Kirkham FJ, Kölbel M, et al. White matter integrity and processing speed in sickle cell anemia. Neurology 2018; 90(23):e2042–e2050.
5. DeBaun MR, Gordon M, McKinstry RC, et al. Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. N Engl J Med 2014; 371(8):699–710.
6. Dlamini N, Saunders DE, Bynevelt M, et al. Nocturnal oxyhemoglobin desaturation and arteriopathy in a pediatric sickle cell disease cohort. Neurology 2017; 89:2406–2412.
7. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006; 31(4):1487–505.
8. Prohovnik I, Hurlet-Jensen A, Adams R, De Vivo D, Pavlakis SG. Hemodynamic etiology of elevated flow velocity and stroke in sickle-cell disease. J Cereb Blood Flow Metab 2009; 29(4):803–10.
9. Kosinski PD, Croal PL, Leung J, et al. The severity of anaemia depletes cerebrovascular dilatory reserve in children with sickle cell disease: a quantitative magnetic resonance imaging study. Br J Haematol 2017; 176(2):280–7.
10. Pagani M, Salmaso D, Sidiras GG, et al. Impact of acute hypobaric hypoxia on blood flow distribution in brain. Acta Physiol 2011; 202(2):203–209.
11. Werring DJ, Brassat D, Droogan AG, et al. The pathogenesis of lesions and normal-appearing white matter changes in multiple sclerosis: A serial diffusion MRI study. Brain 2000; 123(8):1667–1676.
Figures