1636
Evidence for reduced oxygen extraction efficiency in sickle cell anemia patients with cerebral capillary shunting1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Radiology, Harvard Medical School, Boston, MA, United States, 3Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN, United States, 4Pediatrics-Division of Pediatric Neurology, Vanderbilt University Medical Center, Nashville, TN, United States, 5Medicine-Division of Hematology/Oncology, Vanderbilt University Medical Center, Nashville, TN, United States
Synopsis
Venous hyperintense signal in arterial spin labeling (ASL) MRI has been associated with abnormal tissue-capillary water exchange in adults with sickle cell anemia (SCA). We tested the hypothesis that such hyperintense signal is associated with reduced oxygen extraction fraction (OEF) in adults with SCA. Higher categorical scores of shunting were associated with lower OEF in SCA participants with silent infarcts and/or white matter lesions but not in participants without lesions. These findings indicate that venous hyperintense signal in ASL images may reflect impaired abilities of blood to subserve oxygen and may contribute to lesion development in SCA patients.
Introduction
Sickle cell anemia (SCA) is a blood disorder in which production of erythrocytes with hemoglobin-S results in hemodynamic impairment. Compensation for anemia has been demonstrated in individuals with SCA via CBF upregulation1 through increases in arterial blood velocity and relaxation of smooth muscle surrounding arterioles. Given that oxygen exchange efficiency is partially dependent on the rate of blood and water transit through the capillary bed2, high flow in patients with SCA could result in capillary shunting3,4. Paradoxically, shunting effects could lead to suboptimal oxygen delivery to brain tissue despite elevated CBF. Here, we examined whether a relatively novel imaging marker, venous hyperintense signal in arterial spin labeling (ASL) MRI, may be an indicator of capillary shunting and reduced oxygen extraction fraction (OEF) in adults with SCA. Furthermore, we sought to understand how these hemodynamic relationships may be affected in the presence of lesions.Methods
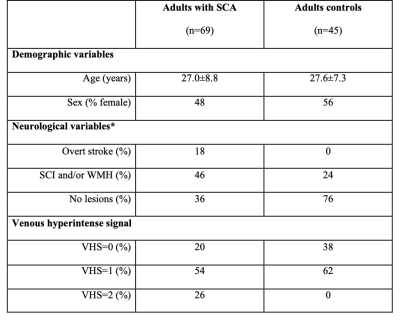
Participants. All participants (n=114) provided informed, written consent for this prospective cross-sectional study. Individuals with SCA (n=69), defined as phenotype hemoglobin SS or Sβ0-thalassemia, and age- and race-matched control volunteers without SCA or sickle trait (n=45) were scanned at 3T (Philips).Experiment. ASL (TR/TE=3675/13 ms; spatial resolution=3x3x7 mm3) MRI data were acquired with pseudo-continuous labeling and post-labeling delay=1900 ms using a multi-slice 2D single-shot echo planar gradient-echo readout. T2-relaxation-under-spin-tagging (TRUST; TR/TE=1978/3.6 ms; spatial resolution=3.4x3.4 mm2; effective echo times=0, 40, 80, 160 ms)5 MRI data were acquired at the level of the superior sagittal sinus for supratentorial OEF measurement. T1-weighted and T2-weighted FLAIR imaging were performed for infarct determination and co-registration. All participants received a neurological examination. Blood hematocrit values were measured through venipuncture. Arterial oxygenation was measured with pulse oximetry.
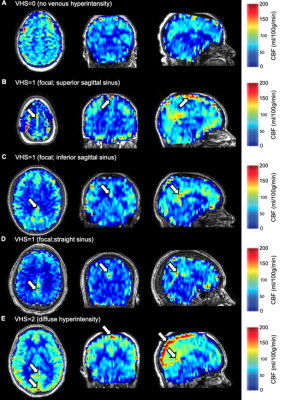
Processing. CBF-weighted images were calculated following pair-wise subtraction and assessed for venous hyperintense signal. Each participant’s images received venous hyperintensity scores (VHS) of 0, 1, or 2 (Figure 1). CBF was quantified from the ASL data using a two-compartment model with subject-specific hematocrit and blood T1 for SCA patients6. OEF was quantified from the TRUST-MRI data separately using models calibrated with human blood over an anemic hematocrit range7,8. Participants were classified by their most significant lesion(s): overt stroke, lesions without an abnormal neurological exam: silent cerebral infarcts (SCI; >3mm in size, visible on two imaging planes) and/or white matter hyperintensities (WMH; <3mm in size), or no lesions/normal MRI of the brain.
Analysis. Wilcoxon rank-sum tests were used to determine whether age, hematocrit, OEF, or CBF differed between groups with venous hyperintense signal absent (i.e., VHS=0) and present (VHS>0). Jonckheere-Terpstra tests were used to determine whether these parameters exhibited monotonic associations with increasing VHS. This step was repeated after categorizing the SCA group according to the three levels of impairment: (i) overt stroke, (ii) lesions without an abnormal neurological exam and (iii) no lesions.
Results
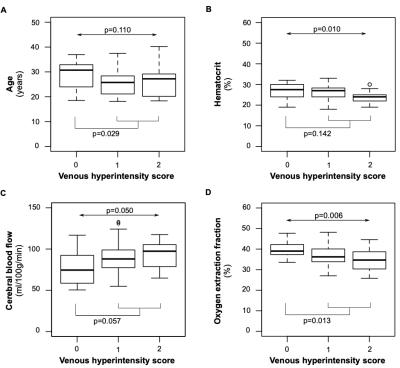
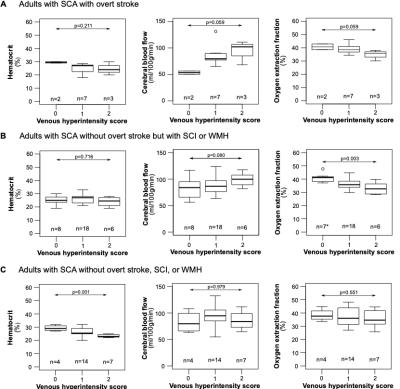
A summary of demographic and neurological findings is shown in Figure 2. We found that a higher degree of VHS was associated with lower hematocrit (Jonckheere-Terpstra p=0.010), higher CBF (Jonckheere-Terpstra p=0.059), and lower OEF (Jonckheere-Terpstra p=0.006) (Figure 3). When separately examining adults with SCA and no overt stroke, we found that a higher degree of VHS was associated with lower OEF only in those with SCI and/or WMH (Jonckheere-Terpstra p=0.003) but not in those without these lesions (Jonckheere-Terpstra p=0.551) (Figure 4).Discussion
In adults with SCA, a higher degree of VHS was associated with (i) lower hematocrit, (ii) higher CBF and (iii) lower OEF. In adults with SCA and no overt stroke, a higher degree of VHS was associated with lower hematocrit in those without SCI or WMH but not in those with these lesions. In the same subgroup of participants, a higher degree of VHS was associated with lower OEF in those with SCI or WMH but not in those without these lesions. This finding may be explained by the rapid water transit through capillaries, secondary to compensatory hyperemic mechanisms in patients with SCA, leading to elevated venous hyperintensities on ASL and also reduced oxygen extraction at the tissue level. This effect appears most significant in patients with prior silent lesions and may indicate inabilities of the microvasculature to fully subserve oxygen to tissue. Observed relationships did not differ based on which (human7 versus sickle8 hemoglobin) model was used for TRUST calibration.Conclusion
In adults with SCA, presence of venous hyperintense signal, as measured using ASL MRI, was associated with lower OEF, as measured with TRUST MRI. In addition, these microvascular flow disturbances associated with lower oxygen extraction fraction were most evident in SCA patients with silent lesions compared with those without lesions.Acknowledgements
This work was supported by the National Institutes of Health [NIH/NINDS grant number 5R01NS078828; NIH/NCATS grant number UL1 TR000445 to Vanderbilt University] and the American Heart Association [grant numbers 14CSA20380466, 19CDA34790002].References
1. Kosinski PD, Croal PL, Leung J, Williams S, Odame I, Hare GM, et al. The severity of anaemia depletes cerebrovascular dilatory reserve in children with sickle cell disease: A quantitative magnetic resonance imaging study. Br J Haematol. 2017;176:280-287
2. Østergaard L, Jespersen SN, Engedahl T, Gutiérrez Jiménez E, Ashkanian M, Hansen MB, et al. Capillary dysfunction: Its detection and causative role in dementias and stroke. Curr Neurol Neurosci Rep. 2015;15:37
3. Juttukonda MR, Donahue MJ, Davis LT, Gindville MC, Lee CA, Patel NJ, et al. Preliminary evidence for cerebral capillary shunting in adults with sickle cell anemia. J Cereb Blood Flow Metab. 2019;39:1099-1110
4. Bush A, Chai Y, Choi SY, Vaclavu L, Holland S, Nederveen A, et al. Pseudo continuous arterial spin labeling quantification in anemic subjects with hyperemic cerebral blood flow. Magn Reson Imaging. 2018;47:137-146
5. Lu H, Ge Y. Quantitative evaluation of oxygenation in venous vessels using t2-relaxation-under-spin-tagging mri. Magn Reson Med. 2008;60:357-363
6. Wang J, Alsop DC, Li L, Listerud J, Gonzalez-At JB, Schnall MD, et al. Comparison of quantitative perfusion imaging using arterial spin labeling at 1.5 and 4.0 tesla. Magn Reson Med. 2002;48:242-254
7. Bush A, Borzage M, Detterich J, Kato RM, Meiselman HJ, Coates T, et al. Empirical model of human blood transverse relaxation at 3 t improves mri t2 oximetry. Magn Reson Med. 2017;77:2364-2371
8. Bush AM, Coates TD, Wood JC. Diminished cerebral oxygen extraction and metabolic rate in sickle cell disease using t2 relaxation under spin tagging mri. Magn Reson Med. 2018;80:294-303
Figures