1618
Angiogenesis following peripheral blood stem cell therapy: evidence from perfusion MRI of patients with ischemic stroke1Department of Radiology, China Medical University Hospital, Taichung, Taiwan, 2Institute of Medical Device and Imaging, National Taiwan University, Taipei, Taiwan, 3China Medical University Hospital, Taichung, Taiwan, 4Department of Automatic Control Engineering, Feng-Chia University, Taichung, Taiwan, 5Department of Diagnostic Radiology, University of Hong Kong, Hong Kong, Hong Kong, 6Graduate Institute of Biomedical Electronics and Bioinformatics, National Taiwan University, Taipei, Taiwan, 7Buddhist Tzu Chi General Hospital, Hualien, Taiwan
Synopsis
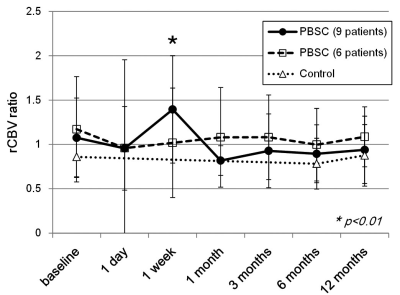
Dynamic susceptibility-contrast perfusion-weighted MRI was performed on 15 chronic stroke patients receiving intracerebral implantation of peripheral blood stem cells (PBSC) at baseline and 6 longitudinal stages after therapy to derive relative cerebral blood volume around the implanted graft relative to contralateral white matter (rCBV ratio), with 15 control patients receiving baseline MRI plus two follow-ups. Nine of the 15 patients (60%) in the PBSC group showed significantly increased rCBV ratio than the baselines (1.39±0.60 versus 1.07±0.44; p < 0.01) at one week after implantation only, preceding functional improvements starting at one month as assessed by NIH stroke scale.
Introduction
Studies on animal models of ischemic stroke highlight the potential of stem cell transplantation for enhancement of angiogenesis to provide an improved environment for neural regeneration 1,2. Evidence from human studies however remains incomplete 3, despite of successful functional recovery in clinical trials 4,5,6. Whether angiogenesis commences as soon as treatment begins and how long the angiogenesis persists also remains unanswered. Therefore the aim of this study was to evaluate the time span of possible presence of angiogenesis and the associated functional improvements after intracerebral implantation of peripheral blood stem cells (PBSC) in patients with chronic ischemic stroke.Methods
15 patients (3 females, 12 males; mean age 50.3±8.1 years; range 36~64 years) receiving intracerebral PBSC implantation were examined with dynamic susceptibility-contrast perfusion-weighted MRI (GE, Signa Excite 3T HDx, Waukesha, WI) using gradient-echo echo-planar imaging (TR/TE = 1500/50 for 1.5 minutes) during the first pass of full dose Gd-contrast at pre-treatment baseline, as well as on 1 day, 1 week, 1 month, 3 months, 6 months, and 12 months after implantation. Another 15 control patients (7 females, 8 males; mean age 56.5±6.4 years; range 41~65 years) received baseline scans, plus 6-month and 12-month follow-ups. Inclusion criteria were: with hemiparetic stroke in one-sided middle cerebral artery territory, 6-60 months after the onset of stroke, National Institute of Health Stroke Scale (NIHSS) scores between 9 and 20, age between 35 and 70 years, no malignant or other major disease. The PBSCs were collected from the patients (autograft) following granulocyte-colony stimulating factor amplification with CD34+ mononuclear cells isolated by magnetic bead separation, followed by intracerebral implantation (3-8 x 106/750 μL) around the infarction cavity rim under MRI-guided navigation. After imaging, relative cerebral blood volume (rCBV) around the implanted graft was compared with contralateral white matter to derive rCBV ratio in each stage. Possible presence of angiogenesis was operationally defined as exhibiting increased rCBV ratio exceeding 15% of the baseline rCBV ratio. NIHSS scores were assessed throughout all follow-up stages.Results
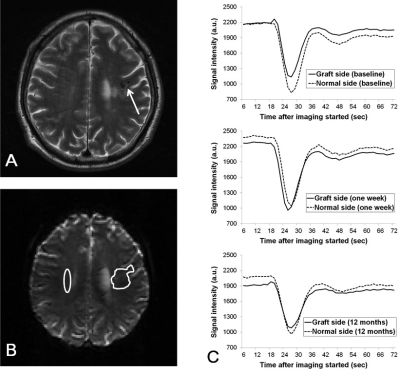
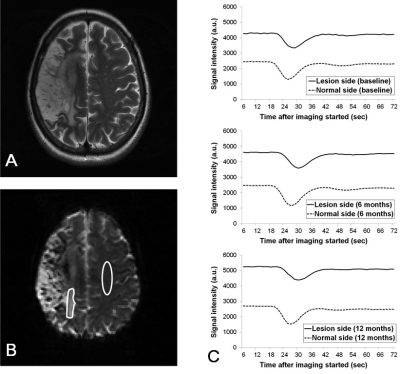
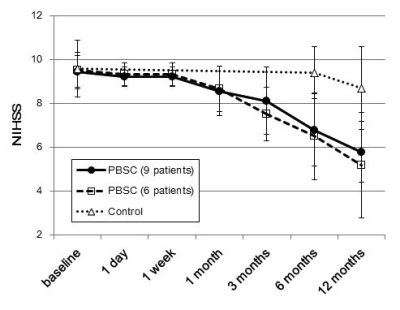
The numbers of patients showing more than 15% increase in rCBV ratio compared with baseline are listed in tabular form in Fig.1, where it is seen that nine PBSC patients demonstrated possible presence of angiogenesis at one week after stem cell implantation. Figure 2 shows the longitudinal rCBV ratio changes for these nine patients, plotted along with that for the remaining six patients in the PBSC group and for the 15 control patients. The nine PBSC patients showed statistically significant increase in rCBV ratios one week after stem cell implantation as compared to the baseline (1.39 ± 0.60 versus 1.07 ± 0.44; p < 0.01). Other than at one week post-implantation for these nine PBSC patients, statistically significant alterations in rCBV ratio were found for neither these PBSC patients at other stages, nor the six remaining PBSC patients and the control subjects. Figures 3 and 4 are the MR images acquired from one PBSC patient and one control patient, respectively, together with the original signal-time curves at the lesion side and the contralateral white matter at different stages. Improvements in the NIHSS scores for PBSC patients commenced at one month after treatment and continued throughout the one-year follow-up period (Fig.5), and were nearly identical between the PBSC patients with and without showing increased rCBV ratio at one week post therapy compared with control.Discussion
The commencement of angiogenesis preceding the improvement of NIHSS scores (at one week and one month post-transplantation, respectively, in our PBSC patients) is in agreement with the possible causal link between angiogenesis and neurogenesis from a large-scale human study examining several angiogenesis biomarkers 7. Our human data that 9 out of 15 PBSC patients showed significantly increased rCBV ratio at one week post PBSC therapy also provided human evidence consistent with past animal investigations in terms of time span 1,2. Limitations of this study include confounding factors such as procedure-related hemorrhage, transient focal interstitial edema, superparamagnetic iron oxide labeled on PBSC to identify the site of injection, which all resulted in uncertainty in rCBV ratio particularly at one day after transplantation.Conclusion
This human study provides imaging evidence of possible presence of angiogenesis around the implanted graft around one week after intracerebral PBSC implantation preceding the improvement of NIHSS scores, which implicates causal relationships between post-therapeutic angiogenesis and neurogenesis.Acknowledgements
Supported in part by research grants from China Medical University Hospital (DMR-101-050), National Science Council (NSC 100-2314-B-039-039), and Ministry of Science and Technology (MOST 105-2314-B-002-094-MY3 to W.C.W. and MOST 105-2221-E-002-142-MY3 to H.W.C.)References
1. Chen J, Zhang ZG, Li Y, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res 2003;92:692-699.
2. Shyu WC, Lin SZ, Chiang MF, et al. Intracerebral peripheral blood stem cell (CD34+) implantation induces neuroplasticity by enhancing beta1 integrin-mediated angiogenesis in chronic stroke rats. J Neurosci 2006;26:3444-3453.
3. Lin CC, Shen WC, Ho YJ et al. Longitudinal perfusion change after intracranial stem cell implantation in chronic. Proc Int Soc Mag Reson Med 2012;2:3094.
4. Steinberg GK, Kondziolka D, Wechsler LR, et al: Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: a phase 1/2a study. Stroke 2016;47:1817-1824.
5. Kalladka D, Sinden J, Pollock K, et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet 2016;388:787-796.
6. Chen DC, Lin SZ, Fan JR, et al. Intracerebral implantation of autologous peripheral blood stem cells in stroke patients: a randomized phase II study. Cell Transplant 2014;23:1599-1612.
7. Navarro-Sobrino M, Rosell A, et al. A large screening of angiogenesis biomarkers and their association with neurological outcome after ischemic stroke. Atherosclerosis 2011;216:205-211.
Figures