1607
Comparison of oxygenation MRI methods with cerebrovascular reactivity in Moyamoya disease using simultaneous [15O]-water PET/MRI1Radiology, Stanford University, Stanford, CA, United States, 2Radiology, Taipei Medical University, Taipei, Taiwan, 3GE Healthcare, Menlo Park, CA, United States, 4Neurosurgery, Stanford University, Stanford, CA, United States
Synopsis
In this [15O]-water PET/MRI study, we compared two non-invasive MRI techniques to measure brain oxygen extraction fraction (OEF) in 10 patients with Moyamoya disease, a steno-occlusive disease of arteries at the base of the brain. Relative OEF from magnetic susceptibility in veins and tissue R2' inversely correlated with [15O]-water PET baseline cerebral blood flow and cerebrovascular reactivity (after vasodilation with acetazolamide). Susceptibility-based OEF in veins was abnormally elevated by 20.4% in regions with severe stenosis compared to healthy tissue. R2' maps showed a smaller OEF elevation (9.6%) that may be confounded by other physiological changes (e.g. blood volume) in disease.
Introduction
In chronic ischemia, oxygen extraction fraction (OEF) in cerebral tissues is pathologically elevated to compensate for lack of perfusion1. Although patient studies and clinical trials in carotid stenosis patients have utilized PET to image OEF prior to by-pass surgery2-3, non-invasive MRI assessment of brain oxygenation requires further evaluation in cerebrovascular patients. This study compares two MRI approaches based on tissue R2’ relaxation4 and quantitative susceptibility in veins5, respectively, to investigate potential OEF abnormalities in patients with Moyamoya disease, a progressive steno-occlusive disorder of arteries at the base of the brain. MRI-derived OEF is compared across different stenosis severities, and correlated with [15O]-water PET measurements of baseline cerebral blood flow (CBF) and cerebrovascular reactivity (CVR) on a simultaneous PET/MRI scanner.Materials and Methods
Simultaneous time-of-flight 3T PET/MRI (GE Healthcare Signa) was acquired in 10 patients with Moyamoya disease (ages 32-62 years, 6 female). Four patients had unilateral disease and the rest had bilateral stenosis or occlusion, with severity assessed by a certified neuroradiologist on MRI angiography. PET imaging of CBF with injection of [15O]-water (550-925 MBq) was performed at baseline and repeated 15 minutes after administration of acetazolamide, a pharmacologic vasodilator. Quantitative CBF maps and vasodilation-induced CBF change (ΔCBF) were calculated by scaling a 2-minute PET frame to the total blood flow measured by phase-contrast MRI (0.375mm in-plane resolution, Venc=100cm/s), acquired simultaneously pre- and post-acetazolamide6.For MRI-based oxygenation measurement at baseline, we acquired multi-parametric maps of R2’, which is proportional to tissue OEF4. These scans included fast spin echo with 8 evenly spaced echoes between 28-122ms for R2 mapping; and multi-echo gradient echo with 10 echoes spaced between 11.1-42.4ms for R2* mapping, both at 0.82x0.82x4.0 mm3 resolution. At baseline, we also acquired high-resolution gradient echo scans (repetition time = 31.5ms, echo time = 17.7ms, 0.41x 0.41x0.70 mm3) with flow compensation for quantitative susceptibility mapping (QSM). A truncated k-division approach was adopted for QSM reconstruction with threshold of 0.12 and correction for point-spread function7.
Average regional values of CBF, ΔCBF, and R2’ were assessed within 10 perfusion territories per hemisphere, after registration of the ASPECTS template8 to the appropriate map. QSM values were also evaluated in major cortical veins draining the ASPECTS perfusion areas, where vessel susceptibility is directly proportional to venous OEF. QSM-derived relative OEF (rOEF) values were normalized to the posterior veins (generally unaffected in Moyamoya disease) to account for global variations across subjects.
Results
Figure 1 shows representative PET/MRI in a 32-year old male Moyamoya patient with severe right middle cerebral artery (MCA) occlusion. Although baseline [15O]-water PET CBF was symmetric, the right MCA territory showed impaired CBF augmentation after vasodilation (green arrows), consistent with poor CVR. QSM maps showed higher density and higher susceptibility values (indicating elevated OEF) for cortical veins in the right hemisphere, and asymmetrically large right lenticulostriate veins. R2’ maps also indicated higher OEF in the right hemisphere, particularly in the white matter. On the other hand, the 33-year old male Moyamoya patient in Figure 2 had normal [15O]-water PET perfusion before and after vasodilation, consistent with preserved CVR. The corresponding QSM maps were largely symmetric in vessel caliber and venous susceptibility, as were the R2’ oxygenation maps.Across patients, [15O]-water PET revealed 22.5% reduction in baseline CBF and 55.6% reduction in ΔCBF for MCA areas with severe stenosis compared to normal territories (Figure 3). ΔCBF was also lower for severe compared to moderate stenosis, which was not observed on baseline CBF. In the same territories, relative OEF assessed by vessel susceptibility was 20.4% higher for severe stenosis relative to normal areas (P=0.007), commensurate with the measured hypo-perfusion. Vein susceptibility (rOEF) also showed a strong inverse correlation with PET baseline CBF and ΔCBF (all P-values < 10-4) (Figure 4). In contrast, R2’ was only elevated by 9.6% in severe stenosis compared to healthy brain areas (P=0.11), in line with a 13% increase observed by a previous MRI study4. Although our R2’ values showed similar inverse relationships with PET perfusion maps, the correlations were weaker, particularly for ΔCBF.
Discussion
Oxygenation MRI revealed areas of abnormal, elevated OEF in Moyamoya disease, which related to the severity of stenosis and inversely correlated with CBF and CVR measured by simultaneous [15O]-water PET. Although R2’ and venous susceptibility MRI provided compatible findings, the susceptibility measurement detected a more robust OEF increase in areas of pathology, with stronger correlation to perfusion deficits. This observation may reflect the fact that vein susceptibility is more specific to deoxyhemoglobin and thus oxygenation status, while R2’ is also sensitive to cerebral blood volume and other tissue properties. More advanced methodology to acquire R2’ maps in a single scan9 and correct for blood volume effects10, as well as improved vessel segmentation tools for QSM11, will further improve the clinical usefulness of MRI oxygenation assessment.Acknowledgements
This study is funded by NIH grants 5K99NS102884-02 and R01EB025220-02.References
1. Leigh R, Knutsson L, Zhou J, van Zijl PC. Imaging the physiological evolution of the ischemic penumbra in acute ischemic stroke. J Cereb Blood Flow Metab (2018): 38(9), 1500-1516.
2. Derdeyn CP, Videen TO, Fritsch SM, Carpenter DA, Grubb Jr. RL, Powers WJ. Compensatory mechanisms for chronic cerebral hypoperfusion in patients with carotid occlusion. Stroke (1999): 30(5), 1019-1024.
3. Grubb RL, Powers WJ, Clarke WR, Videen TO, Adams HP, Derdeyn CP. Surgical results of the carotid occlusion surgery study. Journal of neurosurgery (2013): 118(1), 25-33.
4. Ni WW, Christen T, Rosenberg J, Zun Z, Moseley ME, Zaharchuk G. Imaging of cerebrovascular reserve and oxygenation in Moyamoya disease. J Cereb Blood Flow Metab (2017): 37(4), 1213-1222.
5. Fan AP, Khalil AA, Fiebach JB, Zaharchuk G, Villringer A, Villringer K, Gauthier CJ. Elevated brain oxygen extraction fraction measured by MRI susceptibility relates to perfusion status in acute ischemic stroke. J Cereb Blood Flow Metab (2019): 0271678X19827944.
6. Ishii Y, Thamm T, Guo J, Khalighi MM, Wardak M, Holley D, Gandhi H, Park JH, Shen B, Steinberg GK, Chin FT, Zaharchuk G, Fan AP. Simultaneous phase‐contrast MRI and PET for noninvasive quantification of cerebral blood flow and reactivity in healthy subjects and patients with cerebrovascular disease. J Magn Reson Imag (2019): https://doi.org/10.1002/jmri.26773.
7. Schweser F, Deistung A, Sommer K, Reichenbach JR. Toward online reconstruction of quantitative susceptibility maps: superfast dipole inversion. Magn Reson Med (2013): 69(6), 1581-1593.
8. Kim JJ, Fischbein NJ, Lu Y, Pham D, Dillon WP. Regional angiographic grading system for collateral flow: correlation with cerebral infarction in patients with middle cerebral artery occlusion. Stroke (2004): 35:1340–1344.
9. Stone AJ, Blockley NP. A streamlined acquisition for mapping baseline brain oxygenation using quantitative BOLD. NeuroImage (2017): 147, 79-88.
10. Kaczmarz S, Göttler J, Zimmer C, Hyder F, Preibisch C."Characterizing white matter fiber orientation effects on multi-parametric quantitative BOLD assessment of oxygen extraction fraction." J Cereb Blood Flow Metab (2019): 0271678X19839502.
11. Ward PG, Ferris NJ, Raniga P, Dowe DL, Ng AC, Barnes DG, Egan GF. Combining images and anatomical knowledge to improve automated vein segmentation in MRI. NeuroImage (2018): 165, 294-305.
Figures
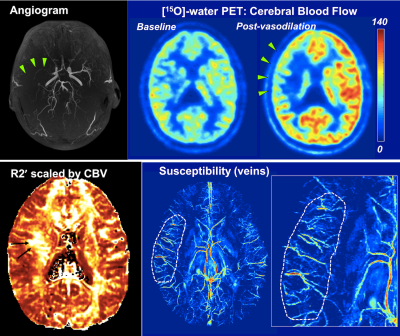
Figure 1. PET/MRI in a 32-y.o. male Moyamoya patient with severe stenosis of the right middle cerebral artery (MCA, green arrows). [15O]-water PET reveals preserved cerebral blood flow (CBF) at baseline, but impaired CBF reactivity after vasodilation in the right MCA territory.
Susceptibility mapping shows dense cortical veins and higher vein susceptibility in the affected hemisphere (dotted), indicating elevated oxygen extraction fraction (OEF). Asymmetric, large right lenticulostriate veins are seen adjacent to areas of elevated white matter OEF on the R2' map (black arrow).
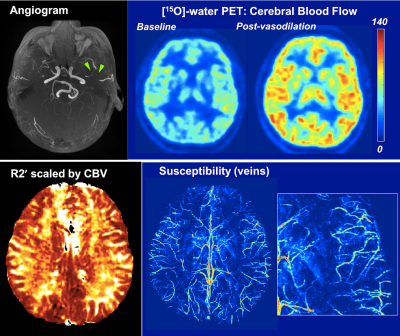
Figure 2. PET/MRI in a 33-y.o. male Moyamoya patient with bilateral involvement and stenosis of left middle cerebral artery (MCA, green arrows). [15O]-water PET reveals normal cerebral blood flow (CBF) at baseline and after vasodilation, consistent with preserved reactivity even in the affected hemisphere.
Correspondingly, the susceptibility map shows symmetric cortical and deep vein density, and normal vein susceptibility values (i.e., normal OEF) across the cortex. The R2' map is also symmetric and indicates preserved tissue OEF in the left hemisphere despite the stenosis.
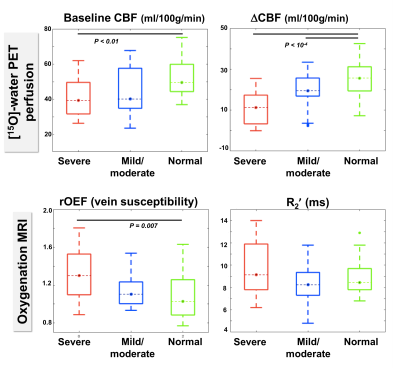
Figure 3. Baseline cerebral blood flow (CBF), flow augmentation (ΔCBF), and oxygenation across patients in the middle cerebral artery (MCA) territory, stratified by severity of MCA stenosis.
Regions with severe stenosis exhibited impaired baseline CBF and ΔCBF compared to normal areas; ΔCBF was also lower for severe compared to moderate stenosis. Relative oxygen extraction fraction (OEF) in veins (from susceptibility mapping) was 20.4% higher in severely stenosed versus healthy areas (P=0.007). R2' maps showed a smaller 9.6% elevation in severely affected areas (P=0.11).
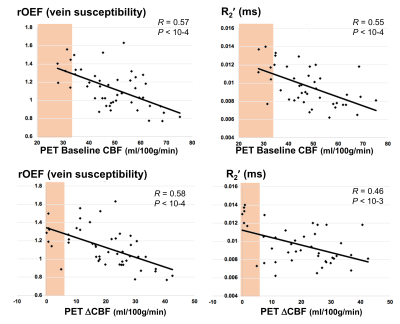
Figure 4. Correlation between perfusion and oxygenation measurements across patients in the middle cerebral artery (MCA) territory. Shaded area indicates cerebral blood flow (CBF) and ΔCBF values that are 2 standard deviations below normal values (in areas without stenosis).
Relative oxygen extraction fraction (OEF), assessed by vein susceptibility MRI, showed strong inverse relationships with PET baseline CBF and ΔCBF. R2' values in tissue also inversely correlated with PET CBF and ΔCBF, albeit with slightly weaker coefficients, especially for cerebrovascular reactivity.