1604
Clinical Feasibility of High-Resolution Intracranial Vessel Wall MRI with Compressed Sensing1Department of Radiology, Severance hospital, Seoul, Republic of Korea
Synopsis
We investigated the clinical feasibility of compressed sensing (CS) in the intracranial vessel wall magnetic resonance imaging (VW-MRI) by applying CS to 3D post-contrast T1-weighted images (T1C). CS enabled larger scan coverage with nearly same scan time, while achieving comparable image quality, normal wall and lesion wall delineation. Furthermore, most T1C with CS provided acceptable quality with regard to overall image quality (84.7%), normal wall delineation (83.3%), and lesion wall delineation (97.8%), which was similar to T1C without CS. Therefore, CS is clinically feasible when applied to the post-contrast intracranial VW-MRI.
Background
Intracranial vessel wall magnetic resonance imaging (VW-MRI) has attracted wide-spread interest in recent years and has been increasingly adopted for visualization of intracranial VW pathology in clinical practice1. High image resolution is a prerequisite for clear visualization of arterial walls and characterization of wall lesions2,3, however, it prolongs scan time. Therefore, scan coverage is compromised to achieve practical scan time while preserving image resolution in the intracranial VW-MRI. Compressed sensing (CS) enables accelerated MRI acquisition using sparse k-space sampling by discarding redundancy in the data acquisition process4-6. Recently, several studies adopted CS in the intracranial VW-MRI and concluded that CS achieved acceptable image quality with reduced image acquisition time7-10. However, the clinical feasibility of CS in intracranial VW-MRI remains unclear, with lack of validation in large patient cohorts.Purpose
The purpose of our study was to investigate the clinical feasibility of CS in the intracranial VW-MRI by applying CS to 3-dimensional post-contrast T1-weighted images (T1C).Materials and Methods
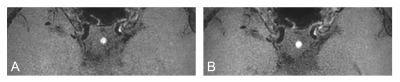
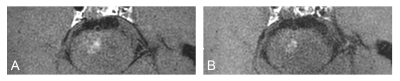
Seventy-two patients who underwent VM-MRI including both T1C with CS (T1C-CS) and without CS (T1C-nonCS) were retrospectively enrolled. T1C-CS enabled larger scan coverage with slightly shorter scan time compared to T1C-nonCS (approximately 7 min vs. 8min). Wall and lumen volumes, signal-to-noise ratio (SNR), and contrast-to-noise ratio (CNR) were measured from normal and lesion sites. Two neuroradiologists independently evaluated overall image quality, degree of normal wall and lesion wall delineation with a four-point scale (scores ≥3 defined as acceptable). Representative cases are shown in figure 1-3. Mcnemar’s test and student t-tests were performed for comparisons.Results
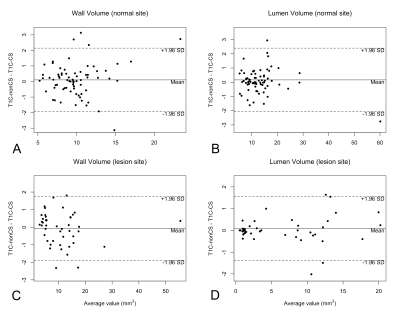
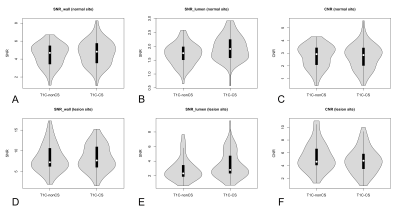
Wall and lumen volumes were not significantly different with T1C-CS or T1C-nonCS (figure 4). In the normal sites, wall SNR and lumen SNR was significantly higher with T1C-CS than with T1C-nonCS (wall SNR: 4.69 ± 1.37 vs. 4.47 ± 1.22, p = 0.013, lumen SNR: 1.94 ± 0.44 vs. 1.71 ± 0.38, p < 0,001). In the lesion sites, wall SNR was similar (8.27 ± 3.09 vs. 8.24 ± 3.28, p = 0.878), while lumen SNR was significantly higher in T1C-CS than in T1C-nonCS (3.50 ± 1.83 vs. 2.85 ± 1.52, p < 0.001), CNR did not differ in normal sites (2.75 ± 0.97 vs. 2.76 ± 0.89, p = 0.907), whereas lower CNR was noted with T1C-CS than with T1C-nonCS in lesion sites (4.77 ± 2.00 vs. 5.39 ± 2.21, p = 0.003). The violin plots presenting the distribution of SNR and CNR from T1C-CS and T1C-nonCS are shown in figure 5. Subjective wall delineation was superior with T1C-nonCS than with T1C-CS (normal wall: 3.51 ± 0.63 vs. 3.38 ± 0.67 [p = 0.019], lesion wall: 3.84 ± 0.33 vs. 3.57 ± 0.49 [p <0.001]), although overall image quality did not differ (3.06 ± 0.63 vs, 3.12 ± 0.60, p = 0.297). The proportions of images with acceptable quality in T1C-CS (83.3 – 97.8%) was similar to T1C-nonCS.Conclusions
CS is clinically feasible when applied to the post-contrast VW-MRI as it enables larger scan coverage with similar acquisition time without compromising image quality.Acknowledgements
No acknowledgement found.References
1. Dieleman N, van der Kolk AG, Zwanenburg JJ, et al. Imaging intracranial vessel wall pathology with magnetic resonance imaging: current prospects and future directions. Circulation 2014;130:192-201. doi:10.1161/circulationaha.113.006919.
2. van der Kolk AG, Zwanenburg JJ, Brundel M, et al. Intracranial vessel wall imaging at 7.0-T MRI. Stroke 2011;42:2478-2484. doi:10.1161/strokeaha.111.620443.
3. Qiao Y, Steinman DA, Qin Q, et al. Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 Tesla. J Magn Reson Imaging 2011;34:22-30. doi:10.1002/jmri.22592.
4. Hollingsworth KG. Reducing acquisition time in clinical MRI by data undersampling and compressed sensing reconstruction. Phys Med Biol 2015;60:R297-322. doi:10.1088/0031-9155/60/21/r297.
5. Jaspan ON, Fleysher R, Lipton ML. Compressed sensing MRI: a review of the clinical literature. The British journal of radiology 2015;88:20150487-20150487. doi:10.1259/bjr.20150487.
6. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med 2007;58:1182-1195. doi:10.1002/mrm.21391.
7. Li B, Li H, Kong H, Dong L, Zhang J, Fang J. Compressed sensing based simultaneous black- and gray-blood carotid vessel wall MR imaging. Magnetic Resonance Imaging 2017;38:214-223. doi:https://doi.org/10.1016/j.mri.2017.01.013.
8. Yuan J, Usman A, Reid SA, et al. Three-dimensional black-blood multi-contrast carotid imaging using compressed sensing: a repeatability study. 2018;31:183-190. doi:10.1007/s10334-017-0640-1.
9. Zhu C, Tian B, Chen L, et al. Accelerated whole brain intracranial vessel wall imaging using black blood fast spin echo with compressed sensing (CS-SPACE). 2018;31:457-467. doi:10.1007/s10334-017-0667-3.
10. Suh CH, Jung SC, Lee HB, Cho SJ. High-Resolution Magnetic Resonance Imaging Using Compressed Sensing for Intracranial and Extracranial Arteries: Comparison with Conventional Parallel Imaging. Korean journal of radiology 2019;20:487-497. doi:10.3348/kjr.2018.0424.
Figures