1597
Optimized T1-weighted dark blood CS-SPACE - assessment of superficial extracranial arteries1Department of Diagnostic and Interventional Radiology, University Hospital Wuerzburg, Wuerzburg, Germany, 2Clinic for Rheumatology and Clinical Immunology, University Hospital Freiburg, Freiburg, Germany, 3Department of Radiology, Medical Physics, University Hospital Freiburg, Freiburg, Germany, 4Rheumatology/Clinical Immunology Department, Internal Medicine II, University Hospital Wuerzburg, Wuerzburg, Germany, 5Siemens Healthcare, Erlangen, Germany, 6University Eye Center, University Hospital Freiburg, Freiburg, Germany, 7Department of Ophthalmology, University Hospital Wuerzburg, Wuerzburg, Germany, 8Department of Neuroradiology, University Hospital Freiburg, Freiburg, Germany
Synopsis
Superficial extracranial arteries are predilection sites for disease manifestation in large vessel vasculitides. We evaluated the suitability of a whole-brain T1-weighted dark blood CS-SPACE sequence, initially developed for intracranial vessel wall imaging, for visualization of vasculitic changes of superficial extracranial arteries. 53 patients were analyzed in a prospective blinded two university medical center trial. First clinical results demonstrate the technique`s applicability for clinical application. Mural thickening and contrast-enhancement of superficial extracranial arteries are clearly visible. The optimized VWI protocol offers potential for assessment of intra- and extracranial disease extent in case of suspected large vessel vasculitis in one single examination.
Introduction
Vasculitides are multisystemic inflammatory diseases with superficial extracranial arteries, especially the superficial temporal and the occipital arteries, as predilection sites in case of large vessel vasculitis [1]. We evaluated a whole-brain T1-weighted dark blood CS-SPACE sequence, optimized for intracranial vessel wall imaging (VWI), regarding its suitability for visualization of vasculitic affection of superficial extracranial arteries.Methods
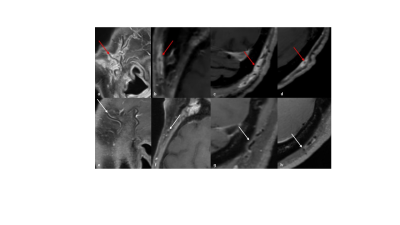
Having developed a sagittal whole-brain T1-weighted dark blood CS-SPACE protocol on a 3T whole-body scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) for optimized intracranial vessel wall imaging, providing an isotropic resolution of 0.55 mm based, we evaluated its suitability for assessment of vasculitic changes of extracranial superficial arteries. Scanning was performed with a 64-channel head coil. A k-space undersampling factor of 0.22 yielded an approximate 5-fold acceleration compared to full k-space sampling and an overall scan time of 5:50 min. Following informed consent, 53 patients were included in a prospective two university medical center trial. Of these, 34 patients had proven GCA (clinically and/or histologically), and 19 were asymptomatic oncological patients undergoing contrast-enhanced MRI to exclude metastatic spread, scanned as controls. The anonymised contrast-enhanced T1-weighted CS-SPACE images were read in blinded fashion by one reader trained in neurovascular imaging. Vessel wall thickening and/or circumferential contrast enhancement was considered vasculitic affection.Results
The optimized whole-brain T1-weighted dark blood CS-SPACE clearly visualizes mural thickening and contrast-enhancement of the superficial temporal and the occipital artery.30 patients of an overall of 53 patients showed contrast-enhancement of at least one of the branches of the superficial temporal artery or the occipital artery. The superficial temporal artery was affected in 20 patients (10 patients bilaterally), the occipital artery was affected in 10 patients (8 patients bilaterally).
27 of 34 patients clinically and/or histologically diagnosed with giant cell arteritis in the further course showed significant contrast-enhancement of at least one of the superficial extracranial arteries. Three of the 16 patients with enhancement of the superficial temporal artery turned out to be asymptomatic oncological patients after unblinding. Sensitivity and specificity of MR-GCA ratings for the detection of GCA diagnosis were 0,79 and 0,84, respectively.
Discussion
MRI has gained in importance and has evolved as one of the preferred and most important diagnostic tools in the diagnosis and management of intra- and extracranial vasculitis [2, 3] and has been proved to be a suitable tool in the initial diagnosis of giant cell arteritis [4]. Since large vessel vasculitides are systemic disorders and display a segmental or rather intermittently distribution pattern of inflamed vessel segments, it is a challenge to capture the whole disease extent. We developed a dedicated T1-weighted dark blood CS-SPACE protocol for the visualization of vasculitic changes of intradural arteries. This sequence turned out to additionally depict vasculitic changes of superficial extracranial arteries with a relatively high reliability, thus offering the potential for capturing intra- and extracranial disease extent simultaneously in one single examination. 3D capacity is beneficial as both intra- and extracranial arteries are thin vessel structures with a tortuous course, rarely fitting standard planes.Conclusion
A high-resolution 3D dark-blood CS-SPACE protocol, initially optimized for intracranial vessel wall imaging clearly visualizes vessel wall thickening and enhancement of the superficial temporal artery and the occipital artery. First clinical results demonstrate its suitability for reliable assessment of superficial extracranial arteries regarding vasculitic affection in large vessel vasculitides. The optimized 3D dark-blood CS-SPACE protocol offers potential for assessment of intra- and extracranial disease extent in case of suspected large vessel vasculitis in one single examination. Further studies are required to establish the technique in clinical Routine.Acknowledgements
Grant support by the Deutsche Forschungsgemeinschaft (DFG) under grant numbers DFG HE 1875/26-2, and BL1132/1-2 is greatly acknowledged.
References
Bley TA, Weiben O, Uhl M, et al. Assessment of the cranial involvement pattern of giant cell arteritis with 3T magnetic resonance imaging. Arthritis Rheum 2005; 52(8): 2470–2477.
H.W. Tan, X. Chen, J. Maingard, C.D. Barras, C. Logan, V. Thijs, H.K. Kok, M.J. Lee, R.V. Chandra, M. Brooks, H. Asadi, Intracranial Vessel Wall Imaging with Magnetic Resonance Imaging: Current Techniques and Applications, World Neurosurg. 2018; 112: 186-198.
Dejaco C, Ramiro S, Duftner C et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 2018; 77: 636–643.
T. Klink, J. Geiger, M. Both, T. Ness, S. Heinzelmann, M. Reinhard, K. Holl-Ulrich, D. Duwendag, P. Vaith, T.A. Bley, Giant cell arteritis: diagnostic accuracy of MR imaging of superficial cranial arteries in initial diagnosis-results from a multicenter trial, Radiology 2014; 273(3): 844-52.
Figures