1596
Optimized Intracranial Vessel Wall Imaging Framework – First clinical results1Department of Diagnostic and Interventional Radiology, University Hospital Wuerzburg, Wuerzburg, Germany, 2Clinic for Rheumatology and Clinical Immunology, University Hospital Freiburg, Freiburg, Germany, 3Department of Radiology, Medical Physics, University Hospital Freiburg, Freiburg, Germany, 4Rheumatology/Clinical Immunology Department, Internal Medicine II, University Hospital Wuerzburg, Wuerzburg, Germany, 5Siemens Healthcare, Erlangen, Germany, 6University Eye Center, University Hospital Freiburg, Freiburg, Germany, 7Department of Ophthalmology, University Hospital Wuerzburg, Wuerzburg, Germany, 8Department of Neuroradiology, University Hospital Freiburg, Freiburg, Germany
Synopsis
The extent of intracranial large artery involvement and its assessment is still part of on-going research. Our study group has developed a whole-brain T1-weighted dark blood CS-SPACE sequence suitable for intracranial vessel wall imaging in large-artery vasculitis combined with a dedicated post-processing tool. 53 patients were analyzed in a prospective blinded two university medical center trial. First clinical results demonstrate the technique`s suitability for clinical application. Mural thickening and contrast-enhancement as well as luminal changes are readily visible. However, certain confounders, especially atherosclerotic vessel wall lesions as well as vasa vasorum still propose major challenges for diagnosing intradural vasculitic affection.
Introduction
MRI has gained significantly in importance for diagnosis and therapeutic monitoring of giant cell arteritis (GCA). In contrast to extracranial involvement of GCA with unambiguous recommendations for assessment of vasculitic changes [1], the extent of intracranial large artery involvement and its assessment is still part of on-going research. We developed a whole-brain T1-weighted dark blood CS-SPACE sequence suitable for intracranial vessel wall imaging (VWI) in large-artery vasculitis combined with a dedicated post-processing tool. Herein, we aim to present our first clinical results.Methods
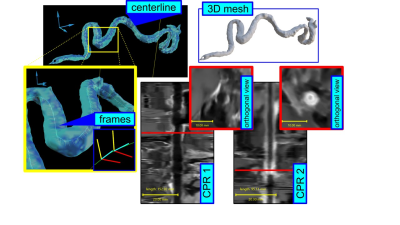
Based on a prior study that revealed the suitability of compressed sensing (CS) MRI for the assessment of cerebral vascular diseases [2], we developed a sagittal whole-brain T1-weighted dark blood CS-SPACE protocol on a 3T whole-body scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) providing an isotropic resolution of 0.55 mm. Scanning was performed with a 64-channel head coil. A k-space undersampling factor of 0.22 yielded an approximate 5-fold acceleration compared to full k-space sampling and an overall scan time of 5:50 min. For better evaluation of the tortuous intracranial vessels, we developed a dedicated post-processing tool, which, after a semi-automated extraction process, enables stretching, straightening and reformatting the originally tortuous vessel for assessment of mural thickening and contrast enhancement of the intracranial arteries. Following informed consent, 53 patients were included in a prospective two university medical center trial. Of these, 34 patients had proven GCA (clinically and/or histologically), and 19 were asymptomatic oncological patients undergoing contrast-enhanced MRI to exclude metastatic spread, scanned as controls. The anonymised contrast-enhanced T1-weighted CS-SPACE images were read in blinded fashion by one reader trained in reading neuroradiology cases regarding vasculitic changes of intracranial arteries. All positive as well as the questionable cases underwent consensus reading with a board-certified radiologist and expert in vasculitis imaging. Vessel wall thickening and/or circumferential contrast enhancement was considered vasculitic affection and focal enhancement < 180° of the vessel`s circumference was considered artherosclerotic plaque.Results
After consensus reading, nine out of 53 patients showed vessel wall enhancement of intradural arteries. The vertebral artery was affected in eight patients (in three cases bilaterally), the internal carotid artery was affected in four patients (in two cases bilaterally) and the middle cerebral artery, basilar artery and posterior cerebral artery were affected in one patient each.Three of the eight patients with enhancement of the vertebral artery turned out to be asymptomatic oncological patients after unblinding.
Discussion
Adequate intracranial VWI is technically demanding as the vessels are precise anatomic structures with a mural thickness of less than 0.5 mm in healthy subjects [3]. Their mostly tortuous course is usually inconsistent with standard MR imaging planes, making the use of 3D techniques with the capacity to multiplanar reconstruction favorable. The combination of high spatial resolution and blood suppression of our optimized 3D dark-blood CS-SPACE sequence enables clear visualization of intracranial artery lumen and wall at the same time. Application of the dedicated post-processing algorithm is mostly automated and takes an average of 5 minutes overall from raw DICOM data set to the final reformats. Stretching and straightening of the tortuous vessel course of the intradural arteries and fade of interfering surrounding structures results in a substantial facilitation of assessment of vessel walls and luminal structures. However, there are certain pitfalls complicating interpretation of VWI findings that have to be kept in mind. Despite the assumption that vessel wall thickening and circumferential enhancement is highly suspicious of vasculitic manifestation, the extent of typically eccentric configured atherosclerotic plaque may affect > 180° of the vessel`s circumference, thus leading to misinterpretation as inflammatory vessel changes. Also, vessel wall enhancement caused by vasa vasorum in the first millimeters after dura perforation may be a confounder in the assessment of intracranial vasculitis. Defined limits for physiological vessel wall enhancement of intradural arteries caused by vasa vasorum would be desirable for a reliable assessment regarding vasculitic affection.Conclusion
The optimized high-resolution 3D dark-blood CS-SPACE VWI protocol combined with the dedicated post-processing tool enables simplified and optimized assessment of tortuous intracranial arteries regarding vasculitic changes. First clinical results demonstrate the technique`s suitability for clinical application. Vessel wall thickening and mural contrast-enhancement as well as luminal changes are readily visible. However, certain confounders, especially atherosclerotic vessel wall lesions as well as vasa vasorum still propose major challenges for diagnosing intradural vasculitic affection in view of a lacking gold standard.Acknowledgements
Grant support by the Deutsche Forschungsgemeinschaft (DFG) under grant numbers DFG HE 1875/26-2, and BL1132/1-2 is greatly acknowledged.
References
Dejaco C, Ramiro S, Duftner C et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 2018; 77: 636–643.
C. Zhu, B. Tian, L. Chen, et al., Accelerated whole brain intracranial vessel wall imaging using black blood fast spin echo with compressed sensing (CS-SPACE), MAGMA Magn Reson Mater Phy., vol. 31(3), pp. 457-67, 2018.
D.M. Mandell, M. Mossa-Basha, Y. Qiao, C.P. Hess, F. Hui, C. Matouk, M.H. Johnson, M.J. Daemen, A. Vossough, M. Edjlali, D. Saloner, S.A. Ansari, B.A. Wasserman, D.J. Mikulis, Intracranial Vessel Wall MRI: Principles and Expert Consensus Recommendations of the American Society of Neuroradiology, AJNR Am J Neuroradiol., vol. 38(2), pp. 218-229, 2017.
Figures
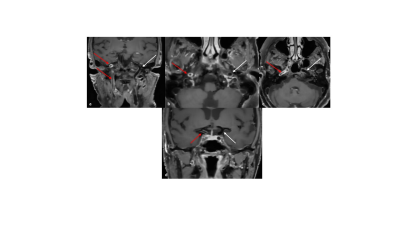
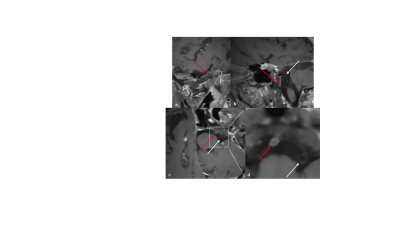
The optimized high-resolution 3D dark-blood CS-SPACE reveals long segmental cervical and petrosal concentric wall enhancement of the right internal carotid artery (a-c, red arrows, target sign), concentric wall enhancement of intradural terminal right ICA segment (d, red arrow) compared to the non-affected left side (a-d, white arrows).