1595
3T intracranial vessel wall MRI reveals that arterial wall thickness increases with reducing hematocrit in patients with sickle cell disease1Vanderbilt University Medical Center, Nashville, TN, United States, 2Mayo clinic, Rochester, MN, United States, 3Vanderbilt University, Nashville, TN, United States, 4Vanderbilt University Medical Center Department of Radiology, Nashville, TN, United States
Synopsis
Sickle cell disease leads to increased risk of intracranial vasculopathy and stroke. Therefore, sensitive radiological indicators of cerebrovascular disease severity are needed to aid in treatment planning. 3T vessel wall imaging MRI studies demonstrated increased basilar artery wall thickness in sickle cell disease patients and inverse relationship between hematocrit and basilar wall thickness likely due to increased blood flow velocities and wall stress. Evaluation of internal carotid artery is difficult possibly due to variable CSF signal suppression in these regions.
INTRODUCTION
Sickle cell disease (SCD) is a monogenetic disorder with a high risk of intracranial vasculopathy and stroke. While hematological measures of anemia and Doppler evaluation of intracranial flow velocity provide coarse metrics of disease severity, only 10-20% of SCD patients have large vessel vasculopathy and as such sensitive radiological indicators of cerebrovascular disease severity that may inform beyond what is discernable on lumenography are needed to provide a more complete perspective of disease severity and aid in treatment planning. We hypothesized that intracranial vessel wall imaging (VWI) performed at the clinical field strength of 3 Tesla (3T), which has already been applied for characterization of intracranial plaque in older adults1 and patients with atherosclerotic intracranial stenosis2,3, could be applied to SCD patients, and specifically that reduced hematocrit and associated elevated flow velocity lead to concentric vessel wall thickening.METHODS
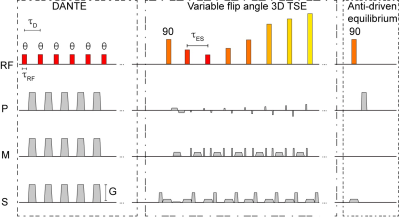
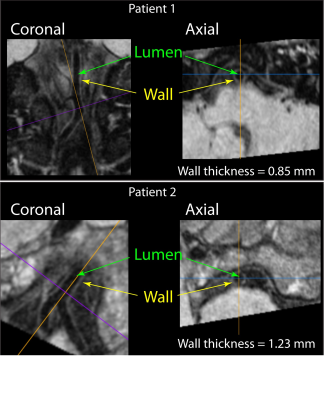
SCD patients (n=83; age (range)=19.4±8.2 (6-39) years; sex=36/47 Male/Female) and age-matched controls (n=38; age (range)=22.2±8.9 (8-39) years; sex=19/19 Male/Female) were consented and underwent a 3T brain MRI with standard anatomical imaging (T1-weighted, T2-weighted, and diffusion weighted imaging), intracranial time-of-flight angiography, and VWI (3D turbo-spin-echo; TR/TE=1500/33 ms; refocusing sweep=40-120°; 0.5x0.5x1 mm). In a subgroup of volunteers (n=38), the VWI protocol was repeated using a delay-alternating-nutation-with-tailored-excitation (DANTE) module with DANTE4 flip angle=8° to evaluate the impact of additional basal cistern CSF suppression on vessel wall contrast-to-noise-ratio (Figure 1). VWI images were reformatted in a plane orthogonal to the vessel course (Figure 2) using guidelines outlined in the literature5 to measure mid basilar artery and bilateral supraclinoid internal carotid arteries (ICA) lumen and wall. Hematocrit was measured in SCD patients within 7 days of imaging. To test the hypothesis that vessel wall thickness was higher in SCD patients versus controls, vessel wall thickness measures were compared using a Student’s t-test. To assess the relationships between vessel wall thickness and clinical indicators of disease severity in SCD patients, first a Pearson’s correlation was applied to evaluate the dependent of vessel wall thickness on hematocrit. Next, regression analyses were performed using vessel wall thickness as the dependent variable and age, sex, vasculopathy extent, and hematocrit as explanatory variables. In all tests, significance criterion was two-sided p<0.05.RESULTS
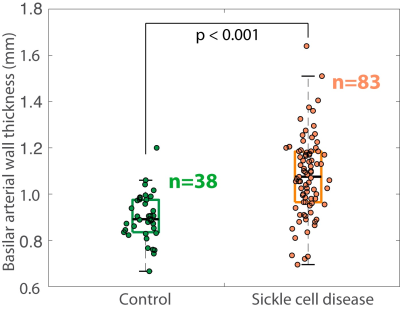
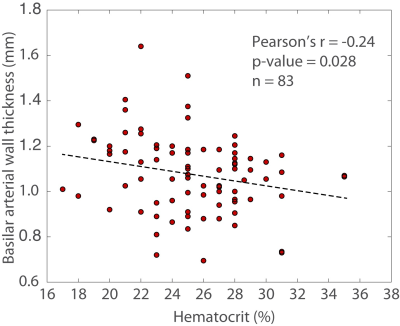
No control participants had evidence of intracranial vasculopathy or infarcts, whereas 12.0% of SCD patients had evidence of intracranial vasculopathy and 48.2% of SCD patients had overt or silent cerebral infarcts. Basilar wall thickness was significantly higher (p<0.001) in SCD patients (1.08 +/- 0.17 mm) compared to age-matched controls (0.90 +/- 0.10 mm) and was inversely related to hematocrit on Pearson’s correlation testing (r=-0.24; p=0.028) (Figures 3-4). On linear regression, an inverse relationship was found between basilar wall thickness and hematocrit (p=0.046) after controlling for age, sex, and vasculopathy. These finding were not significant when considering ICA wall thickness, however trends for significance were observed at the two-sided p-value of 0.10 – 0.20 when ICA thickness was utilized as the vessel wall measurement of interest.DISCUSSION
This study utilized an intracranial 3T VWI protocol with parameters within guideline ranges2 in SCD patients and age- and sex-matched control volunteers. Reduced hematocrit was found to inversely correlate with basilar vessel wall thickness in SCD patients, consistent with increased flow velocities and wall stress leading to concentric wall thickening. ICA vessel walls did not meet criteria for significance, possibly due to more variable CSF signal suppression in these regions as has been described6. When a subset (n=38) of scans was repeated with additional CSF suppression from DANTE preparation modules, measurements were not significantly different. However, the vessel wall was deemed to be more difficult to interpret due to lower contrast-to-noise-ratio. These findings are consistent with vessel wall damage persisting in the absence of overt vasculopathy visible on MRA in patients with SCD, and suggest that vessel wall damage as a result of reduced hematocrit and associated higher and more turbulent flow profiles can manifest beyond simple luminal narrowing. This may suggest that vascular regulation and oxygen extraction at the microvascular level may be impaired due to vessel wall damage.CONCLUSION
Vessel wall thickness, which can be measured using non-invasive 3T VWI MRI at the spatial resolution of 0.5-1 mm, may provide an additional marker of cerebrovascular impairment in SCD patients.Acknowledgements
No acknowledgement found.References
1. Harteveld A, van der Kolk A, van der Worp H, et al. High-resolution intracranial vessel wall MRI in an elderly asymptomatic population: comparison of 3T and 7T. Eur Radiol. 2016;27(4):1585-1595.
2. Mandell D, Mossa-Basha M, Qiao Y, et al. Intracranial Vessel Wall MRI: Principles and Expert Consensus Recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol. 2016;38(2):218-229.
3. Qiao Y, Guallar E, Suri F, et al. MR Imaging Measures of Intracranial Atherosclerosis in a Population-based Study. Radiology. 2016;280(3):860-868.
4. Li L, Miller K, Jezzard P. DANTE-prepared pulse trains: A novel approach to motion-sensitized and motion-suppressed quantitative magnetic resonance imaging. Magn Reson Med. 2012;68(5):1423-1438.
5. Cogswell P, Lants K, Davis L, et al. Vessel wall and lumen characteristics with age in healthy participants using 3T intracranial vessel wall magnetic resonance imaging. J Magn Reson Imaging. 2019;50(5):1452-1460.
6. Cogswell P, Siero J, Lants K, et al. Variable impact of CSF flow suppression on quantitative 3.0T intracranial vessel wall measurements. J Magn Reson Imaging. 2018;48(4):1120-1128.
Figures