1594
Intracranial aneurysms (IA) segmentation from 3D TOF-MRA and black-blood MRI (BB-MRI) using a deep convolutional neural network1Center for Biomedical Imaging Research, Department of Biomedical Engineering, Tsinghua University, Beijing, China, 2The Johns Hopkins University, Baltimore, MD, United States, 3Department of Interventional Neuroradiology, Beijing Neurosurgical Institute and Beijing Tiantan Hospital, Capital Medical University, Beijing, China
Synopsis
In this work, we proposed an automatic segmentation algorithm of intracranial aneurysms from dual input 3D TOF-MRA and black-blood MRI (BB-MRI) using a deep convolutional neural network to study its clinical potential for assisting intracranial aneurysm detection. The positioning of an intracranial aneurysm can benefit from the TOF-MRA, and the BB-MRI image can be used to accurately trace its boundary and measure its size. The average Dice coefficients are 0.69 and 0.73 for the TOF-MRA and the BB-MRI images, respectively.
Introduction
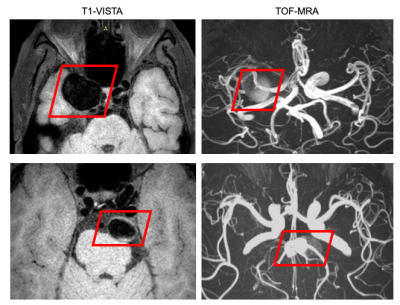
Intracranial aneurysms (IAs) are dilatations of cerebral arteries1, and subarachnoid hemorrhage caused by rupture of an IA is fatal2. Detection of the IA before it ruptures may prevent death3. However, since IA detection is challenging even for experienced radiologists4, reliable computer-aided detection tools are desired. Among the development of advanced imaging techniques, TOF-MRA is particularly attractive since it can provide valuable information regarding the size and shape of an IA5. Although it is suitable for approximate positioning of aneurysms, it underestimates aneurysm size and has measurement error6. Compared with TOF-MRA, BB-MRI using T1-weighted volumetric isotropic turbo spin echo acquisition (T1-VISTA) imaging achieves better accuracy for determining the boundary of an IA and measuring its size6, but it is hard to distinguish the sections of aneurysms from normal vessels in BB-MRI due to the similar round black structure. To combine their advantages, we aimed to segment IAs from dual-contrast 3D TOF-MRA and black-blood MRI (BB-MRI) using a deep convolutional neural network and thus study its clinical potential for assisting IA detection.Methods
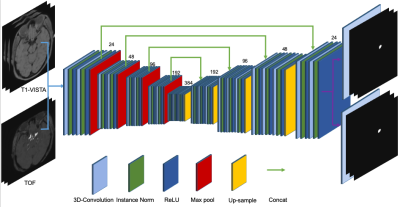
Ninety patients with unruptured IAs were recruited in this study. Two readers determined the presence and locations of aneurysms. Manual voxel-wise segmentation of the aneurysms was performed by the first reader using the open-source annotation software ITK-SNAP (www.itksnap.org). The position of an aneurysm was roughly identified according to the TOF image, and the boundary was delineated separately on both images. The second reader confirmed the location of the aneurysm by reviewing DICOM series, original reports, including gold standard images (Digital Subtraction Angiography), as well as clinical histories of the patients. Three delineations with poor alignment between the two contrasts were removed from the cohort. Forty-six images were randomly selected as the training data, and the remaining forty-four images were used as the testing data. TOF-MRA and T1-VISTA were taken as two-channel input to the network, as shown in Figure.1. The network outputs two probability maps corresponding to the IA delineations on both images, respectively. The segmentation was formulated as a voxel-wise binary classification problem. In the preprocessing step, inhomogeneity correction was performed using the N4 algorithm7, the TOF-MRA image was rigidly registered to the T1-VISTA image, and they were further resampled to 1 mm isotropic resolution and cropped or zero-padded to a spatial shape of 192 x 192 x 96. A modified 3D U-net8 (Figure.2) was used in this work. Instance normalization9 was used instead of batch normalization. Dropout10 with probability 0.2 was used. Two convolution layers with kernel size 1 x 1 x 1 were used for the segmentations, corresponding to the two contrasts at the end of the network, respectively. Right-left flipping and random rotation, scaling, and deformation were used to augment the training data. The whole images were used as the input and the mini-batch size was 4. Adam11 optimizer was used with learning rate 0.001. One minus the Dice coefficient was used as the loss for each of the outputs and the final loss was the average between these two. 380 epochs were used to train the network.Results
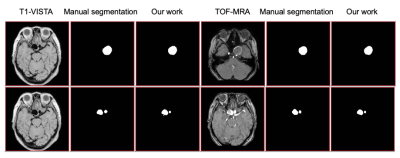
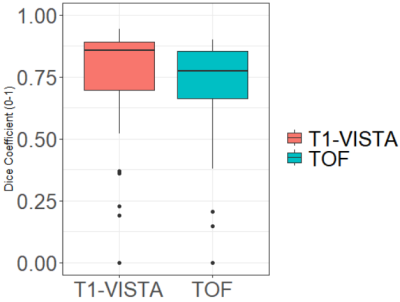
Figure.3 shows the segmented results of aneurysms in dual-contrast images. Probability maps were converted into hard segmentations based on a 0.5 threshold. Figure.4 shows the Dice coefficients between the manual delineation and the network output of the testing images for both contrasts. The average Dice coefficients are 0.73 and 0.69 for the BB-MRI and the TOF-MRA images, respectively.Discussion and Conclusions
In this work, we proposed an automatic segmentation algorithm of IAs from dual-contrast images based on a 3D U-net. The positioning of an IA can benefit from the TOF-MRA, and the T1-VISTA image can be used to accurately trace its boundary and measure its size. For future investigation, our approach might contribute to an improvement of aneurysm detection in a clinical setting, and the performance should be compared with human readers.Acknowledgements
No acknowledgement found.References
1. Bonneville F et al. Neuroimaging Clinics of North America, 2006, 16(3):371.
2. Juhana Frösen et al. Translational Stroke Research 2014; 5(3):347-356.
3. Sasidharan G M et al. Neurology India, 2015, 63(1):96.
4. Okahara M et al. Stroke. 2002 ;33 :1803–8.
5. Mario C et al. European Journal of Radiology, 2013, 82(12):E853-E859.
6. Zhu C et al. American Journal of Neuroradiology, 2019,40(6):960-966
7. Nicholas J et al. IEEE Trans Med Imaging. 2010 Jun; 29(6): 1310–1320.
8. Çiçek, et al. International Conference on Medical Image Computing and Computer-Assisted Intervention. 2016.
9. Ulyanov D et al. IEEE. 4105–4113.
10. Tompson J et al. IEEE, 2015.
11. Kingma D et al. Computer Science, 2014.
Figures