1591
Feasibility of Dark Blood Susceptibility-Weighted Imaging1Center for Neuroscience and Regenerative Medicine, NIH/USU, Bethesda, MD, United States, 2Radiology and Imaging Sciences, NIH, Bethesda, MD, United States
Synopsis
First-order flow compensation has been widely used in MRI. While it works for visualization of vessels and CSF, the residual flow effects can impact the vessels visualization in some applications, such as minimum intensity projection, which is often used in detecting cerebral microhemorrhages on SWI images. When applying minIP, slight offset of spatial registration, and residual flow dephasing from acceleration and pulsatility can manifest as segments of hypointensities, compounding the difficulties in microhemorrhage detection. By including flow sensitization gradients in SWI sequence to suppress flow signal, dark vessels are delineated at correct spatial locations.
INTRODUCTION
In magnetic resonance imaging, proton movement, such as those in blood vessels or cerebrospinal fluid, during playout of magnetic field gradients results in erroneous phase accumulations at echo time (TE). These accumulations are dependent on proton velocity, acceleration, and pulsatility. Without proper correction, they lead to incorrect frequency and phase encoding in k-space, which in turn manifest as flow artifacts in image domain. Flow compensation1,2 employs additional gradients to null gradient moments so that the accumulated phase of moving protons is correct at TE, and thus no flow artifacts in the resultant image. For first-order gradient moment nulling (GMN), one additional gradient is needed to compensate for the velocity of protons; for second-order GMN, two gradients are needed to compensate for acceleration; and for third-order GMN, three gradients are needed for pulsatility.Adding gradients in a pulse sequence requires extending minimum TR and possibly minimum TE. Therefore, in practice, only first-order flow compensation is implemented and in general it works adequately that most flow artifacts are suppressed and the bulk part of blood vessels are correctly spatially encoded. Ideally, signal from blood in vessels would be of uniform intensity and spatially registered. However, incomplete flow compensation due to flow acceleration and pulsatility leads to incomplete restoration of signal and somewhat inaccurate spatial encoding of tissue. This is clearly seen on, for example, minimum intensity projection (minIP) whish is often applied to detect cerebral microhemorrhages5 or vascular thrombus on SWI3,4 images. Because of the above, arteries in particular can appear irregular or frankly inrerrupted into segments and thus misinterpreted as vascular stenoses or as microhemorrhage.
METHODS
To enable flow sensitization, a pair of magnetic field gradients were added to readout, phase-encoding, and slice-select directions of a gradient-recalled echo (GRE) pulse sequence. The two gradients were the same but of opposite polarities to prevent altering spatial encoding.Healthy volunteers were scanned under an IRB approved protocol (NCT00001711) using a vendor-provided GRE and the custom GRE-FlowSens pulse sequences on a Siemens Biograph mMR 3T MRI system (Siemens, Erlangen, Germany). Contrast parameters were TR = 27 ms, TE = 20 ms, and flip angle = 15°. Geometric parameters included image matrix of 256 × 256 × 40, and voxel size of 0.9 × 0.9 × 4 mm. A total of 3 sets of images were obtained: GRE without and with flow compensation (GRE-FlowComp), and GRE-FlowSens.
RESULTS
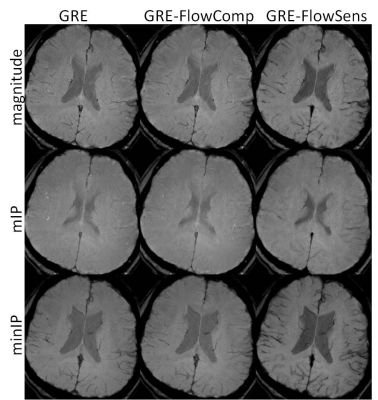
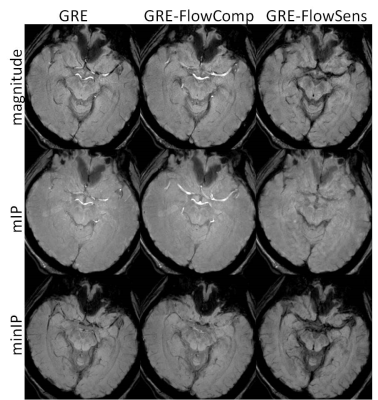
In Figure 1, magnitude, mIP, and minIP images from GRE, GRE-FlowComp, and GRE-FlowSens were compared. While flow compensation spatially registers the bulk part of blood vessels to correct locations, mis-registration occurs when it was incomplete as indicated by the arrow. The blood vessel structure was better delineated in GRE-FlowSens images. Residual flow dephasing can manifest as hypointense segments in minIP, as shown in Figure 2. In certain contexts such as traumatic brain injury, these segments can mimic cerebral microhemorrhages.DISCUSSION
We developed a dark blood SWI sequence by adding flow sensitization gradients in GRE sequence, which dephases blood signals and thus better delineate blood vessels. It is shown that this sequence may be able to reduce false positive of hypointense pathology, such as microhemorrhages. The tradeoff of having flow sensitization gradients is that, while they dephase signals of flowing protons, the additional gradients will make the sequence more sensitive to patient motion. Further optimization is needed in this regard.CONCLUSION
Dark blood SWI using a flow sensitization strategy results in dark arteries which better conform to the anatomic structure of the vaculature by minimizeing the artifacts resulting from incomplete flow compensation. This strategy may improve the utility of SWI for assessing the vasculature and minimize the generation of artifactual microhemorrhage.Acknowledgements
This study was supported by the Department of Defense in the Center for Neuroscience and Regenerative Medicine and the Intramural Research Program of the National Institutes of Health.References
1 A. D. Elster, 'Motion Artifact Suppression Technique (Mast) for Cranial Mr Imaging: Superiority over Cardiac Gating for Reducing Phase-Shift Artifacts', AJNR Am J Neuroradiol, 9 (1988), 671-4.
2 E. M. Haacke, and G. W. Lenz, 'Improving Mr Image Quality in the Presence of Motion by Using Rephasing Gradients', AJR Am J Roentgenol, 148 (1987), 1251-8.
3 E. M. Haacke, S. Mittal, Z. Wu, J. Neelavalli, and Y. C. Cheng, 'Susceptibility-Weighted Imaging: Technical Aspects and Clinical Applications, Part 1', AJNR Am J Neuroradiol, 30 (2009), 19-30.
4 E. M. Haacke, Y. B. Xu, Y. C. N. Cheng, and J. R. Reichenbach, 'Susceptibility Weighted Imaging (Swi)', Magnetic Resonance in Medicine, 52 (2004), 612-18.
5 R. N. K. Nandigam, A. Viswanathan, P. Delgado, M. E. Skehan, E. E. Smith, J. Rosand, S. M. Greenberg, and B. C. Dickerson, 'Mr Imaging Detection of Cerebral Microbleeds: Effect of Susceptibility-Weighted Imaging, Section Thickness, and Field Strength', American Journal of Neuroradiology, 30 (2009), 338-43.
Figures