1588
Changes in resting state connectivity and brain metabolites during a season of collegiate basketball: A pilot study1Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, GA, United States, 2Department of Biomedical Engineering, Georgia Institute of Technology and Emory University, Atlanta, GA, United States, 3Department of Medicine, Philadelphia College of Osteopathic Medicine, Suwanee, GA, United States, 4Department of Sports Medicine, Emory University School of Medicine, Atlanta, GA, United States
Synopsis
Sports-related traumatic brain injuries are difficult to diagnose and prognose due to a lack of standardized metrics. Furthermore, there has been limited research on the effects of repeated sub-concussive and sub-clinical injuries over time. In this study, we characterized changes in resting state connectivity and brain metabolites over a season of collegiate basketball in athletes without a diagnosed concussion. We observed significant changes in resting state connectivity and brain metabolites as a function of game time played. No changes in plasma inflammatory markers were observed over time, suggesting that brain changes were not driven by systemic inflammation.
Introduction
Diagnosing traumatic brain injuries in collegiate athletes is challenging due to heterogeneous presentation, lack of recovery biomarkers, and non-standardized diagnostic measures. While previous research that characterized brain changes during contact sports largely focused on concussion, evidence suggests that repeated sub-concussive and sub-clinical injuries may contribute to long-term damage.1,2 Our goal was to identify changes in resting state connectivity, brain metabolites, and systemic inflammation during a season of collegiate basketball in athletes with repeated sub-concussive injuries but without amnesia or loss of consciousness.Methods
Healthy male subjects were recruited from a single collegiate basketball team after obtaining written informed consent (mean ± standard deviation age: 21 ± 2 years old). Magnetic resonance imaging (MRI), spectroscopy (MRS), and blood draws were collected before the first game (pre-season; n=7), mid-season (n=6), and after the final game (post-season; n=5) (Figure 1). MR data was acquired on a 3T Siemens PrismaFIT MR scanner using a 32-channel head coil. Resting state functional MRI (rsMRI) (8.25-minute multiband; TR/TE=750/37ms; voxels=2.50×2.50×3.15 mm3, flip angle=54°, ETL=88), T1-weighted MPRAGE, GRE field maps, and single-voxel PRESS (TR/TE=1700/30ms; averages=128; bandwidth=1200 Hz; complex data points=1024; 2-cm isotropic voxels in the posterior cingulate and left and right frontal white matter) were acquired at each time point. Game time played between MR scans was calculated for each subject using the U.S. National Collegiate Athletic Association official record. Whole blood was collected via venipuncture the same day as MRI, centrifuged to isolate plasma, and stored at -80⁰ C. Plasma inflammatory markers (interleukin (IL)-10, IL-6, IL-8, tumor necrosis factor-alpha, and C-reactive protein) were quantified with electrochemiluminescence assays (Meso Scale Diagnostics). Preprocessing of rsMRI data included slice timing, field map, and motion correction; EPI/T1/MNI coregistration and normalization; and smoothing and denoising (linear detrending, bandpass filtering to exclude signals outside 8-90 mHz, and deconvolution of the six motion parameters, CSF, white matter signals, and first-order derivatives of CSF and white matter signals from individual voxels using the CompCor method3). All functional and structural data were manually inspected to confirm registrations and pre-whitening efficacy and subjected to bivariate seed-to-voxel and region of interest (ROI)-to-ROI analyses. ROIs consisted of functionally- and anatomically defined 1-mm MNI-space atlas regions included in the CONN toolbox (MATLAB 2018, MathWorks).4 MRS data was analyzed with LCModel5 and metabolites were normalized to total creatine. Changes in inflammatory marker and metabolite concentrations over time were determined using the Kruskal-Wallis test. A generalized linear model was used to determine associations between rsMRI connectivity and metabolites with game time played, controlling for time delay between the last game played and the MR scan. Significance was determined at p≤0.05.Results and Discussion
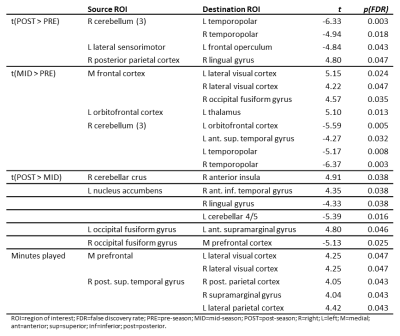
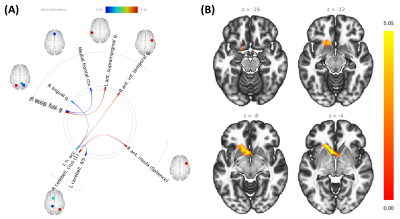
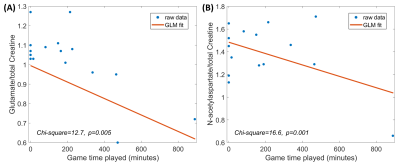
Changes in ROI-to-ROI connectivity and seed-to-voxel connectivity were observed throughout the season and as a function of minutes played (Table 1). As injuries are more common during games and injury reports are qualitative, game time played was used as a metric for injury. Game time played was positively correlated with bilateral medial prefrontal-lateral visual cortex connectivity, and connectivity between the posterior aspect of the right-hemisphere superior temporal gyrus, right-hemisphere posterior parietal cortex, right-hemisphere supramarginal gyrus, and lateral parietal cortex. Mid-season versus pre-season contrast revealed increased connectivity between left thalamus and left orbitofrontal cortex and between the medial prefrontal cortex and right-hemisphere fusiform gyrus, medial prefrontal cortex and lateral visual cortex bilaterally; decreased connectivity was observed between the posterior fossa of the cerebellum and the superior temporal and temporopolar cortices. Post-season/pre-season contrast was characterized by decreased cerebellar/temporopolar connectivity, decreased connectivity between left frontal operculum and left lateral sensorimotor areas, increased connectivity between right lingual and right posterior parietal cortices (Figure 2a), and greater involvement of the anterior cingulate (Figure 2b). Post-season/mid-season contrast revealed stronger positive correlations between nucleus accumbens and right anterior inferior temporal gyrus, and stronger negative correlations between the nucleus accumbens/left cerebellum and nucleus accumbens/right lingual gyrus post-season relative to mid-season (Figure 3a). Additionally, stronger positive correlations were observed between cerebellum and insula, as well as left-hemisphere fusiform and supramarginal gyri, and stronger anticorrelations between right-hemisphere fusiform and medial frontal cortex. Subjects also exhibited greater recruitment of the left nucleus accumbens/caudate nucleus post-season relative to mid-season (Figure 3b). Myo-inositol concentrations in the left frontal white matter significantly differed between groups (p=0.037), and post-hoc tests revealed significant decreases post-season compared to mid-season (p=0.017). Right frontal white matter glutamate and N-acetylaspartate, normalized to total creatine, were negatively associated with game time played (p≤0.05) after controlling for time differences between the last game played and the MR scan (Figure 4). We did not observe significant changes in plasma inflammatory markers, suggesting that systemic inflammation was not the primary driver for observed changes in connectivity or brain metabolite concentrations.Conclusions
Over a single season of collegiate basketball, we observed significant changes in resting state connectivity and brain metabolite concentrations as a function of game time played. Systemic inflammatory markers did not change over time. Further studies in a larger sample size will explore potential underlying mechanisms responsible for the observed brain changes including neuroinflammation, learning, and injury type.Acknowledgements
MR experiments were facilitated by the Emory Center for Systems Imaging Core. Immunoassays were performed by the Emory Multiplexed Immunoassay Core with funding from the Georgia Clinical & Translational Science Alliance (NIH UL1TR002378).References
1. Blennow K et al. Traumatic brain injuries. Nat Rev Dis Primers. 2016;17(2):16084.
2. Saigal R and Berger MS. The long-term effects of repetitive mild head injuries in sports. Neurosurgery. 2014; 75:S149-155.
3. Behzadi Y et al. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37(1):90-101.
4. Whitfield-Gabrieli S and Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125-141.
5. Provencher, SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672-679.
Figures