1585
Detection of brain microstructural alterations in epilepsy using DTI and mean apparent propagator (MAP)-MRI1Deptpartment of Radiology, SUN YAT-SEN Memorial Hospital, SUN YAT-SEN University, Guangzhou, China, 2MR Scientific Marketing, Siemens Healthcare, Guangzhou, China, 3MR Scientific Marketing, Siemens Healthineers, Shanghai, China, 4Shanghai Key Laboratory of Magnetic Resonance, East China Normal University, Shanghai, China
Synopsis
We investigated the clinical feasibility of characterizing brain tissue microstructure in epilepsy with mean apparent propagator (MAP)-MRI, and compared with diffusion tensor imaging (DTI) using whole-brain voxel-based analyses. Results demonstrated that the MAP-MRI microstructural parameters could potentially provide more sensitive clinical biomarkers than conventional DTI techniques due to increased pathophysiological specificity in microstructural changes detection.
Introduction
Epilepsy is a common neurological disease, which can be interfered by antiepileptic drugs in the early stage, but more than 30% of patients will develop drug resistance. At present, neurosurgery is an effective treatment for refractory epilepsy in clinical, so it is a great significance to accurately locate the epileptic focus before operation. EEG is widely used to roughly detect epileptic focus with abnormal potential, but it cannot provide accurately location (1). Magnetic resonance imaging (MRI) is a more advanced methodology for lesion detection, especially advanced diffusion imaging, which provide better visualization of brain tissue microstructures in vivo(2). This study aimed to evaluate the performance of mean apparent propagator (MAP)-MRI and diffusion tensor imaging (DTI) in detecting brain microstructural alterations of epilepsy using whole-brain voxel-wise analyses.Materials and Methods
A total of 19 patients with epilepsy (22.2±14.8 years old, 10 males and 9 females) and 20 healthy controls (HC) (38.8±15.5 years old, 12 males and 8 females) underwent MR scans on a 3T MR scanner (MAGNETOM Skyra; Siemens Healthcare, Erlangen, Germany) with a 20-channel head coil. Three-dimensional (3D) sagittal T1-weighted magnetization prepared rapid acquisition with gradient echo (MPRAGE) images (repetition time (TR)/echo time (TE), 1800 ms/2.32 ms; flip angle, 8°; number of excitations (NEX), 1; 0.9-mm effective slice thickness; 160 slices; matrix, 256 × 256; field of view (FOV), 230 × 230 mm.) were acquired. Diffusion spectrum imaging (DSI) using q-space half coverage diffusion mode with on-line dynamic field correction (TR/TE, 4900 ms/93 ms; flip angle, 90°; NEX,1; voxel dimension of 2 × 2 × 4 mm3; 32 slices; matrix, 110 × 110; FOV, 220 × 220 mm; ) was measured along 128 gradient directions with maximum b-value at 2000 s/mm2.For data post-processing, the MAP and DTI models were fitted by a software called NeuDiLab, which developed in-house based on an open-resource tool DIPY (Diffusion Imaging In Python, http://nipy.org/dipy). The parameters derived from MAP model included return-to-origin probability (RTOP), return to axis probability (RTAP), return to plane probability (RTPP), mean squared displacement (MSD) and Q-space inverse variance (QIV), and from DTI model included fractional anisotropy coefficient (FA), mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD) . To explore each parameter by whole-brain voxel-wise analyses, all the diffusion parameters were spatially normalized to MNI space using affine and nonlinear registration with several steps by advanced normalization tools (ANTs; http://stnava.github.io/ANTs/). The b=0 image of DWI data from each individual was affine transformed onto its 3D-T1 image to obtain affine transformation matrix, and all 3D-T1 images were nonlinearly registered to MNI templateto acquire the spatial transformation matrix, then all the diffusion parameters were sequentially transfer into MNI space using the combination of affine and spatial transformation matrix. After spatial normalization, the voxel-based whole brain analysis were conducted. The quantitative parameters of DSI and DTI models, using “two-sample t-test” design with age as a covariate, were analyzed to investigate the brain abnormalities in epilepsy by Statistical Parametric Mapping 12 (SPM12; http://www.fil.ion.ucl.ac.uk/spm/) software running in MATLAB. For all SPM analyses, only results that met the following criteria were deemed significant: a seed threshold of p < 0.001 with false discovery rate (FDR) correction (p=0.05).Results
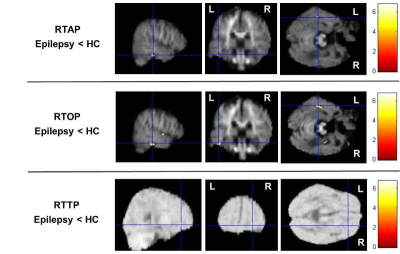
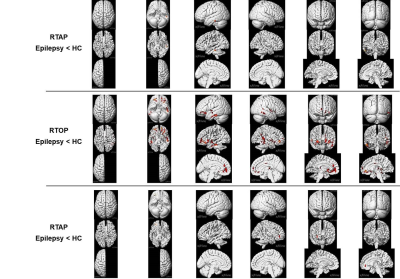
Compared with the healthy controls, the epilepsy group presented significant RTAP reductions in the temporal lobe (p=5×10-6) and RTPP reductions in the right frontal lobe (p=5.5×10-6). Additionally, significant widespread RTOP decreases (bilateral temporal Lobe, bilateral frontal lobe, right parietal lobe, bilateral Sub-lobar, Extra-Nuclear, right insula, right thalamus, right limbic lobe and cingulate gyrus) were found in the epilepsy group (p=2.5×10-4), but other parameters of DTI and MAP-MRI models do not showed significant differences (Figure 1, 2).Discussion and Conclusion
Our results showed that RTAP, RTOP and RTPP detected significant brain tissue changes between epilepsy patients and the healthy controls, which suggested that the MAP-MRI parameters can reflect intrinsic micro-anatomical changes and showed higher sensitivity and specificity in lesion detection than DTI parameters. MAP-MRI, which build a powerful analytical framework without the assumption of a Gaussian spin displacement distribution, can more accurately and comprehensively characterize noninvasively structural and architectural features of brain tissues than DTI(3).Acknowledgements
No acknowledgement found.References
1.Daichi Sonea, Noriko Sato. Abnormal neurite density and orientation dispersion in unilateral temporal lobe epilepsy detected by advanced diffusion imaging. NeuroImage: Clinical 20 (2018) 772–7822.
2. Alia Lemkaddema, Alessandro Daducci. Connectivity and tissue microstructural alterations in right and left temporal lobe epilepsy revealed by diffusion spectrum imaging. NeuroImage: Clinical 5 (2014) 349–3583.
3.Alexandru V. Avram , Joelle E. Sarlls , Alan S. Barnett. Clinical feasibility of using mean apparent propagator (MAP) MRI to characterize brain tissue microstructure. NeuroImage 2015
Figures