1584
Altered cerebral functional connectivity asymmetry in unilateral mesial temporal lobe epilepsy
Xu Zhao1, Zhiqiang Zhou2, and Wenzhen Zhu1
1Radiology department, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, 2Anesthesiology department, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
1Radiology department, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, 2Anesthesiology department, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Synopsis
The human brain is structurally and functionally asymmetrical. The asymmetry of mesial temporal lobe epilepsy (MTLE) were not clarified yet. This study analyzed the functional asymmetry of MTLE by comparing the functional connectivity (FC) of the left to the right hemisphere directly. The results showed reduction of asymmetrical areas in MTLE and a different asymmetrical features in left and right MTLE. The reduced FC asymmetry in MTLE may play an important role in the cognitive impairment of MTLE. The different asymmetrical features in left and right MTLE may hold the potential for differentiating left from right MTLE.
INTRODUCTION
Both structural and functional asymmetries have been well-documented in the human brain and thought to be a central principle of nervous system architecture and shape the functional organization of most cognitive systems1,2. Mesial temporal lobe epilepsy (MTLE) is one of the most common types of refractory focal epilepsy in adults and hippocampal sclerosis (HS) is the hallmark of most MTLE3. Asymmetric patterns were observed of the structural and functional alterations in MTLE, that is, these abnormalities were more obvious in the hemisphere ipsilateral to the epileptogenic focus than the contralateral hemisphere4,5. What is more, some studies showed that MTLE with left HS (LMTLE) presented different structural and functional abnormalities from MTLE with right HS (RMTLE) 6-8. Another important characteristic is that LMTLE showed differential effects on the cognitive functions versus RMTLE as well9,10. The above evidences give us some hints that whether HS located in left hemisphere or right hemisphere matters in the pathogenesis mechanisms of MTLE. We hypothesized that the different presences between LMTLE and RMTLE might have something to do with the functional asymmetric features of human brain. Therefore, in this study, we used rs-fMRI to analyze the functional asymmetric features of MTLE by comparing the FC of the left hemisphere to the right hemisphere directly. We hypothesized that the functional asymmetries of MTLE would be altered compared to HC, and the functional asymmetries would distinctly affect the left and right MTLE.METHODS
23 patients with unilateral HS (LMTLE group, n=12; RMTLE group, n=11) and 23 healthy controls with matched age and gender were recruited. After preprocessing, the data were parcellated into 100 cortical and subcortical regions, including 50 paired region of interest (ROIs) located in left and right hemispheres respectively using the FSL Harvard-Oxford Atlas. In order to estimate the FC asymmetry of each ROI of the three groups, we calculated the right FC (rFC) and left FC (lFC) of each ROI respectively. rFC and lFC are the absolute values of total correlation coefficients strongly correlated (threshold at r>0.25) with the ROI in the right hemisphere and left hemisphere, respectively. A two-sample paired t-test was conducted for the rFC and lFC of each ROI to analyze the FC asymmetry, and the significance level was set at P<0.05. In order to analyze the FC asymmetry among the three groups, we calculated asymmetry index (AI) using the formula $$$AI=100*(rFC-lFC)/[(rFC+lFC)/2]$$$. For the statistical comparison of AIs among the three groups, a univariate ANOVA was used, and a post hoc analysis with Bonferroni correction was included for multiple comparisons and to determine the direction of AI differences among groups. Linear correlation analysis was further performed between AIs of significant lateralized ROIs and epilepsy onset age and epilepsy duration.RESULTS
In the HC group, among the total 100 ROIs, FC of 49 ROIs (49/100) showed asymmetrical features, including 30 ROIs (30/100) of rightward asymmetry (RA) and 19 ROIs (19/100) of leftward asymmetry (LA). In the patient groups, the number of ROIs showed asymmetrical features were reduced. In LMTLE, 36 ROIs (36/100) showed asymmetrical features, including 12 RA and 24 LA. In RMTLE, 23 ROIs (23/100) showed asymmetrical features, including 17 RA and 6 LA. The between-group analysis of AIs for the three groups showed significant differences of 24 ROIs which mostly located in bilateral insular cortex (IC), temporal lobe, frontal lobe and limbic system. Our study showed a negative correlation between the AI of right pars opercularis FC and the epilepsy onset age in LMTLE (r = -0.621, P = 0.031). In addition, a positive correlation between the AI of left temporal pole FC and the epilepsy duration in RMTLE (r = 0.633, P = 0.036).DISCUSSION
The hemispheric asymmetry of certain functions such as language, spatial attention and memory in the human brain was thought to be beneficial for functioning1,11,12. It is reported that distinct functions and a division of labor between the left and right hemispheres could improve overall cognitive ability and performance13. Thus, the reduced FC asymmetry in patients with MTLE might play an important role in the cognitive impairment of MTLE. the ROIs showed abnormal asymmetric features involved both temporal lobe and extra-temporal zones supported the theory that MTLE is a network disease 14. Many studies have shown consistent IC involvement in MTLE and the IC was assumed to be linked to the somesthetic and emotional symptoms in MTLE 10,15. Thus the strengthened FC of IC presented in this study could be related with the abnormal emotional symptoms such as fear and somesthetic aura such as epigastric aura experienced by MTLE. Many studies showed bilateral limbic system impairments in TLE 16,17, but few paid attention to the asymmetric alterations of limbic system in TLE. The asymmetric alterations of bilateral limbic system in our study provided evidences that the bilateral limbic systems play different roles in the communication and compensatory mechanism associated with the bilateral sides of brain.CONCLUSION
The asymmetrical features in MTLE were reduced, which may play an important role in the cognitive impairment of MTLE. The different asymmetrical features in left and right MTLE may hold the potential for differentiating left from right MTLE.Acknowledgements
The authors are grateful to Prof. Pan Lin and Dr. Huicong Kang in Department of Neurology, Tongji Hospital, Tongji Medical College of HUST for helpful in postprocessing and diagnosing of MTLE.References
1 Toga, A. W. & Thompson, P. M. Mapping brain asymmetry. Nature Reviews Neuroscience 2003; 4(1): 37-48. 2 Ocklenburg, S., Friedrich, P., Güntürkün, O. et al. Intrahemispheric white matter asymmetries: the missing link between brain structure and functional lateralization? Reviews in the Neurosciences 2016; 27(5). 3 Cendes, F., Andermann, F., Gloor, P. et al. Atrophy of mesial structures in patients with temporal lobe epilepsy: cause or consequence of repeated seizures? Annals of neurology 1993; 34(6): 795-801. 4 Pereira, F. R., Alessio, A., Sercheli, M. S. et al. Asymmetrical hippocampal connectivity in mesial temporal lobe epilepsy: evidence from resting state fMRI. BMC neuroscience 2010; 11: 66. 5 Bonilha, L., Rorden, C., Halford, J. J. et al. Asymmetrical extra-hippocampal grey matter loss related to hippocampal atrophy in patients with medial temporal lobe epilepsy. Journal of neurology, neurosurgery, and psychiatry 2007; 78(3): 286-294. 6 de Campos, B. M., Coan, A. C., Lin Yasuda, C. et al. Large-scale brain networks are distinctly affected in right and left mesial temporal lobe epilepsy. Human brain mapping 2016; 37(9): 3137-3152. 7 Besson, P., Dinkelacker, V., Valabregue, R. et al. Structural connectivity differences in left and right temporal lobe epilepsy. Neuroimage 2014; 100: 135-144. 8 Li, H., Fan, W., Yang, J. et al. Asymmetry in cross-hippocampal connectivity in unilateral mesial temporal lobe epilepsy. Epilepsy research 2015; 118: 14-21. 9 Kim, H., Yi, S., Son, E. I. et al. Differential effects of left versus right mesial temporal lobe epilepsy on Wechsler intelligence factors. Neuropsychology 2003; 17(4): 556-565. 10 Doucet, G., Osipowicz, K., Sharan, A. et al. Hippocampal functional connectivity patterns during spatial working memory differ in right versus left temporal lobe epilepsy. Brain connectivity 2013; 3(4): 398-406. 11 Badzakova-Trajkov, G., Haberling, I. S., Roberts, R. P. et al. Cerebral asymmetries: complementary and independent processes. PloS one 2010; 5(3): e9682. 12 Habib, R., Nyberg, L. & Tulving, E. Hemispheric asymmetries of memory: the HERA model revisited. Trends in cognitive sciences 2003; 7(6): 241-245. 13 Gotts, S. J., Jo, H. J., Wallace, G. L. et al. Two distinct forms of functional lateralization in the human brain. Proceedings of the National Academy of Sciences of the United States of America 2013; 110(36): E3435-3444. 14 Pittau, F., Grova, C., Moeller, F. et al. Patterns of altered functional connectivity in mesial temporal lobe epilepsy. Epilepsia 2012; 53(6): 1013-1023. 15 Bouilleret, V., Dupont, S., Spelle, L. et al. Insular cortex involvement in mesiotemporal lobe epilepsy: a positron emission tomography study. Annals of neurology 2002; 51(2): 202-208. 16 Liacu, D., Idy-Peretti, I., Ducreux, D. et al. Diffusion tensor imaging tractography parameters of limbic system bundles in temporal lobe epilepsy patients. Journal of magnetic resonance imaging : JMRI 2012; 36(3): 561-568. 17 Concha, L., Beaulieu, C. & Gross, D. W. Bilateral limbic diffusion abnormalities in unilateral temporal lobe epilepsy. Annals of neurology 2005; 57(2): 188-196.Figures
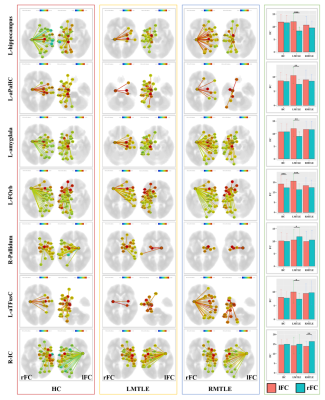
Figure 1. The
left three columns represents the right functional connectivity (rFC) and left
functional connectivity (lFC) of the HC, LMTLE and RMTLE groups respectively.
The right column showed the FC asymmetry of each ROI of the three groups (*P<0.05;
**P<0.01; ***P<0.001). L-aPaHC, left anterior
division of parahippocampal gyrus; L-FOrb, left frontal orbital cortex; L-aTFusC,
left anterior division of temporal fusiform cortex; R-IC, right insular cortex.
HC, healthy controls; LMTLE, left mesial temporal lobe epilepsy; RMTLE, right
mesial temporal lobe epilepsy.
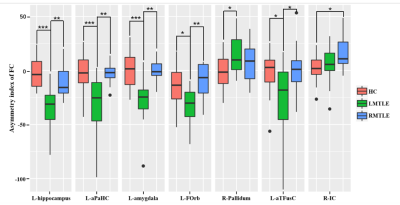
Figure
2. The asymmetry index (AI) of functional connectivity (FC) differences of the
ROIs
among HC, LMTLE and RMTLE. Red represents FC in
HC; green, patients with LMTLE; blue, patients with RMTLE (*P<0.05;
**P<0.01; ***P<0.001). L-aPaHC,
left anterior division of parahippocampal gyrus; L-FOrb, left frontal
orbital cortex; L-aTFusC,
left anterior division of temporal fusiform cortex; R-IC, right insular cortex.
HC, healthy controls; LMTLE, left mesial temporal lobe epilepsy; RMTLE, right
mesial temporal lobe epilepsy.
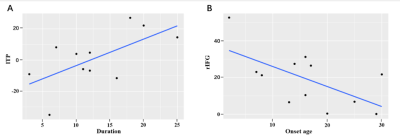
Figure
3. Correlation between the asymmetry index (AI) of functional connectivity (FC)
and clinical variables of epilepsy. (A) represents a positive correlation
between the AI of left temporal pole (lTP) FC and the epilepsy duration in the
RMTLE group (r = 0.633, P = 0.036). (B) represents a negative
correlation between the AI of right pars opercularis of inferior frontal gyrus
(rIFG) FC and the epilepsy onset age in the LMTLE group (r = -0.621, P =
0.031). LMTLE, left mesial temporal lobe epilepsy;
RMTLE, right mesial temporal lobe epilepsy.