1582
Diffusion Tensor Metrics Abnormalities in Developing brain: A Comparative Study Between Epilepsy and Simple Febrile Seizure aged 6-60 months
Abdelkareem Salimeen Mustafa1, Xianjun Li1, Miaomiao Wang1, Congcong Liu1, Martha Singh1, Mengxuan Li 1, Xiaocheng Wei2, and Jian Yang1,3
1First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China, Xi’an, China, 2MR Research China, GE Healthcare, China, Beijing, China,, Beijing, China, 3The Key Laboratory of Biomedical Information Engineering, Ministry of Education, Department of Biomedical Engineering, School of Life Science and Technology, Xi’an Jiaotong University, Xi’an, Shaanxi 710049, People’s Republic of China,, Xi’an, China
1First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China, Xi’an, China, 2MR Research China, GE Healthcare, China, Beijing, China,, Beijing, China, 3The Key Laboratory of Biomedical Information Engineering, Ministry of Education, Department of Biomedical Engineering, School of Life Science and Technology, Xi’an Jiaotong University, Xi’an, Shaanxi 710049, People’s Republic of China,, Xi’an, China
Synopsis
Epilepsy is a common neurological disorder spread worldwide. Simple febrile seizures (simple FS) is convulsive disorder associated with fever. However, little known about diffusion changes during the developing brain. The study aimed was to assess the diffusion changes in epilepsy and simple FS at aged of 6 to 60 months, using diffusion tensor imaging (DTI). Through inter-group comparisons, fraction anisotropy (FA) decreased, and radial diffusivity (RD) increased in epileptic children compared to simple FS and control. These results suggested DTI is sensitive method in diagnosis delayed myelination in epilepsy, while simple FS have good prognosis due to different pathophysiological mechanism.
INTRODUCTION
Childhood epilepsy is the most common neurological disorder in the world (1). It is spread among 3rd world countries (2). Pediatrics epilepsy is heterogeneous in its presentation, with different etiologies. Psychiatric and neurobehavioral are common complications of this disease (2). Around 2.4 million people were diagnosed every year (3). Childhood epilepsy is common during developing brain and its susceptible to seizures (4). Failure to control epileptic fits may lead to brain damage and cognitive decline (3,5). One-third of childhood epilepsy has resistance to antiepileptic drugs (6). Febrile seizures (FS) is a common convulsive disorder associated with fever. Simple febrile seizures (Simple FS) is generally considered as a benign entity and not causing brain damage or cognitive impairment (7). Simple FS referred to that generalized seizures resolve spontaneously in less than 15 min, and do not recurrent within 24-hours or in the same illness (8). White matter (WM) integrity changes during developing brain had great clinical importance in understanding neurodevelopmental disturbance, which originates from early abnormal structural and functional maturation (9). Overall, there is a gap knowledge in the pathophysiology and progression of epilepsy during developing brain. Diffusion tensor imaging (DTI) can acquire quantitative information about microstructure integrity changes. However, few studies address the diffusion changs in epilepsy children and compare with simple FS during developing brains. Therefore, study aimed was to evaluate the WM integrity in epilepsy and simple FS children during developing brain for this pilot we use DTI and tract-based spatial statistics (TBSS).MATERIALS AND METHODS
All Children parents gave written informed consent; the study was approved by the local institutional review board. We retrospectively evaluate 33 epileptic children, 26 simple FS children, and 28 control. The epileptic and simple SF children were diagnosed by pediatric neurologists. MRI examinations were performed at the ages of 6–60 months using a 3T scanner (Signa HDxt, GE Healthcare, Milwaukee, Wisconsin, USA) with an 8-channel head coil. We acquired T2weighted sequence (TR/TE, 4200ms/120ms; matrix, 256*256; slice thickness, 2.5mm; FOV,240mm) and T2FLAIR sequence (TR/TE, 8600ms/165ms; matrix, 288*224; slice thickness, 5mm; FOV,240 mm). The following are DTI parameters: 30 gradient directions; b values = 0 and 600 s/mm2; TR/TE = 11000/69.5ms; slice thickness = 2.5 mm without spaces; FOV = 240 mm; and matrix size = 128 × 128. FMRIB software library (FSL, www.fmrib.ox.au.uk/fsl) was used for processing DTI data. We obtain FA maps after the brain extraction and the eddy correction. We utilize linear and non-linear image registrations for alignment of the FA maps of all subjects. FA maps of all subjects were registered to the target map. The mean FA skeleton and FA map were created. The aligned FA map of each subject was projected into the mean FA skeleton (threshold = 0.15). We perform voxel-wise statistical analysis to assess between groups. We extracted the regional values from the areas with significant differences demonstrated by TBSS. All variables were analyzed by using Chi-square test in the SPSS software (Version 21.0; IBM, Armonk, New York, USA). Variables were analyzed by using nonparametric test. P-values less than 0.05 were considered statistically significant.RESULTS
There was no significant difference between epileptic children, simple FS and control groups in age, sex and gestational age (Table1). TBSS analysis demonstrates a significant decrease FA secondary to increased radial diffusivity (RD) for the epileptic children, compared with simple FS and control, while there was no significant difference between simple SF and control (Figure1, Table2&3).DISCUSSION
In epilepsy patients, FA decreased in most of brain WM secondary to increase RD, it’s not findings in simple FS (Figure1, Table2&3). These findings demonstrated that the extent of diffusion changes in developing brain epilepsy. These two patterns maybe influenced by different pathophysiological mechanisms and etiologies between the two diseases (5,7). However, DTI metrics provide quantitative measures of water diffusion (10). Decrease FA and increase bulk RD in WM are indicated microstructural integrity changes, the possible result in delayed myelination, as well as fibers densities changes (11). The changes in association, commissural and projection fibers may lead to motor, sensory, and cognitive decline.CONCLUSION
The contribution of this work DTI method can provide an evidence that delayed myelination maybe first diagnostic biomarker appears in epileptic children during developing brain. Simple FS children may have good prognosis due to different pathophysiological mechanism and etiologies of these diseases.Acknowledgements
The study was supported by the National Key Research and Development Program of China (2016YFC0100300), National Natural Science Foundation of China (No.81171317, 81471631, 81771810), the 2011 New Century Excellent Talent Support Plan of the Ministry of Education, China (NCET-11-0438), the Clinical Research Award of the First Affiliated Hospital of Xi’an Jiao Tong University (No. XJTU1AF-CRF-2015-004) , the Fundamental Research Funds for the Central Universities (xjj2018265), the Fundamental Research Funds of the First Affiliated Hospital of Xi'an Jiaotong University (2017QN-09).References
1. Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: A meta-analytic approach. 2010;51(5):883–90. 2. Newton CR, Garcia HH. Epilepsy 2 Epilepsy in poor regions of the world. Lancet [Internet]. 2012;380(9848):1193–201. Available from: http://dx.doi.org/10.1016/S0140-6736(12)61381-6 3. Koh S. Role of Neuroinflammation in Evolution of Childhood Epilepsy. 2018;33(1):64–72. 4. Dupuis N. Inflammation and Epilepsy in the Developing Brain : Clinical and Experimental Evidence. 2015;21:141–51. 5. Pitkänen A, Lukasiuk K. Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol. 2011;10(2):173–86. 6. Henshall DC, Hamer HM, Pasterkamp RJ, Goldstein DB, Kjems J, Prehn JHM, et al. MicroRNAs in epilepsy: pathophysiology and clinical utility. Lancet Neurol. 2016;15(13):1368–76. 7. Eun BL, Abraham J, Mlsna L, Kim MJ, Koh S. Lipopolysaccharide potentiates hyperthermia-induced seizures. Brain Behav. 2015;5(8):1–10. 8. Ma L, McCauley SO. Management of Pediatric Febrile Seizures. J Nurse Pract. 2018;14(2):74–80. 9. Ota M, Sato N, Maikusa N, Sone D. Whole brain analyses of age ‑ related microstructural changes quantified using different diffusional magnetic resonance imaging methods. Jpn J Radiol. 2017;35(10):584–9. 10. Alexander AL, Lee JE, Lazar M, Field AS. Diffusion Tensor Imaging of the Brain. Neurotherapeutics. 2008;4(3):316–29. 11. Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002; 15:435–55.Figures
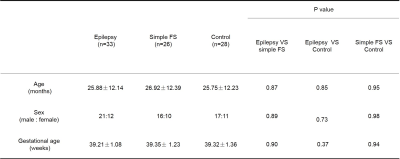
Table1: Participant
clinical and demographic data.
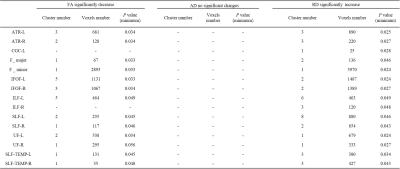
Table2. Cluster and
voxel number with significant differences between control and epilepsy on FA,
AD, and RD based on ROI findings.
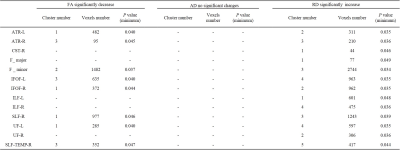
Table 3. Cluster and voxel number with significant differences
between simple FS and epilepsy on FA, AD and RD based on ROI findings.
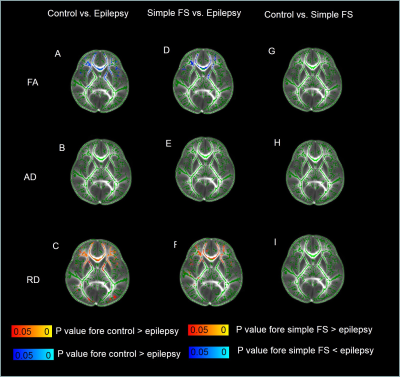
Figure 1.
Inter-group comparisons by using TBSS.