1572
R2-based brain iron measurements in patients with iron overload – a retrospective analysis of selected brain regions1Department of Diagnostic and Interventional Neuroradiology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, 2Department of Diagnostic and Interventional Radiology and Nuclear Medicine, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, 3UCSF Benioff Children’s Hospital Oakland, Oakland, CA, United States, 4Department of Pediatric Hematology and Oncology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, 5Imperial College, London, United Kingdom, 6Department of Radiology, University of Texas Southwestern Medical Center, Dallas, TX, United States
Synopsis
This study investigates the measurement of iron overload in patients using R2 MRI in selected brain regions.
Introduction
In certain diseases requiring repeated blood transfusions, such as ß thalassemia and sickle cell disease, iron overload is a well-known phenomenon. Chelation treatment has proved instrumental in counteracting the cytotoxic damage caused by excessive iron accumulation and has led to decreased mortality in certain patient populations [1]. Despite this, vital organ iron toxicity of chronically transfused patients still presents a serious clinical problem, requiring vigilant therapy observation and optimization [2]. Extensive studies on the effects of high iron concentrations on the liver and heart have been performed, leading to considerable progress in the development of diagnostic and therapeutic strategies. In recent years, MRI has emerged as an accurate, widely available, and non-invasive tool for iron quantification, and has been incorporated into routine clinical use for the evaluation of cardiac and hepatic iron [3,4]. Its transferability to other vital organs that may not be easily accessible is becoming increasingly apparent. For example, pituitary iron levels assessed by MRI is currently an active area of research [5]. In patients suffering from ß thalassemia major, pituitary iron accumulation can have dramatic effects, leading to hypogonadism, reduced fertility, and delayed development of secondary sexual characteristics. The extent to which iron overload also occurs in other regions of the brain, as well as in other patient groups, has yet to be thoroughly investigated, with few studies addressing this subject [6,7]. The aim of this study was to employ MRI-R2 sequences to examine the iron content of selected brain structures in patients undergoing repeated blood transfusions. We hypothesize that certain regions preferentially accumulate iron, potentially serving as a quantitative marker of disease. The results could aid in the clinical validation and technical standardization of MRI for routine monitoring, greatly benefiting patients suffering from transfusional siderosis.Methods
Our collective consisted of 27 patients suffering from transfusion-dependent thalassemia (TDT, 15), Diamond-Blackfan anemia (DBA, n = 7), or sickle cell disease (SCD, n = 5), as well as 7 healthy controls. Laboratory, transfusion and chelation treatment data, as well as comorbidities, were documented. Each patient received cardiac, hepatic, and pituitary MRI-R2*/-R2 scans for iron assessment. Beyond the pituitary, R2-based iron levels of the bilateral caudate head, bilateral thalamus, corpus callosum, and pons were retrospectively determined in the sagittal scans from a 1.5T scanner (Symphony, Siemens, Erlangen, Germany) with an 8-element coil using multi-slice turbo spin echo sequences with the following parameters: TR/TE: 2500ms/15-120ms, flip angle 180°, 11 slices at a thickness of 3mm, 0.3mm gap. Anatomical structures were manually delineated in the sagittal plane and analyzed using CMRtools software (v. 2013, Cardiovascular Imaging Solutions, London, UK). The resulting R2 levels for each patient group and region were compared to those of the healthy controls, as well as to reference data from the literature.Results
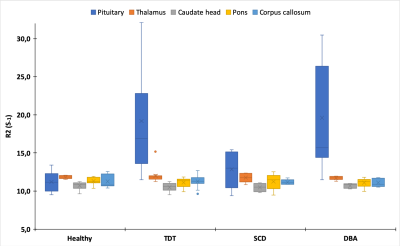
A total of 34 participants were included in this pilot study. 15 (44%) were female and the average age was 24 years (range 12 - 67). R2-based iron measurements showed that patients suffering from TDT and DBA exhibited significantly higher levels of pituitary iron when compared to healthy controls (p ≤ 0.001 and p ≤ 0.01, respectively), with an overall range of 11.5 - 41.2s-1. Interestingly, this effect was neither observed for the SCD patients, nor for the other studied regions of the brain (p ≥ 0.13, Figure 1). Indeed, apart from the pons, which was found to be slightly higher than the age-matched reference values found for R2 measurements (approx. 11s-1 vs. 8.6s-1 p < 0.0001), all other structures were found to be within the normal range [8].Discussion
Our preliminary results of significantly elevated levels of pituitary iron, as measured by R2, in the TDT and DBA patient subgroups confirm previous studies [9]. The fact that the SCD subgroup was not similarly affected could be explained by the fact that these patients also often do not exhibit high levels of cardiac or endocrine iron overload [10], likely due to lower transfusion frequency. It was, however, surprising that none of the other examined brain regions had higher than normal iron concentrations, regardless of underlying illness. Studies employing other MRI-based methods of iron assessment, such as R2* and QSM, have found conflicting results, particularly with regard to the caudate nucleus and thalamus, as well as to the correlation between cardiac, pancreatic, and serum iron levels [6,7,11]. It is worth noting that, due to the retrospective design of this study initially intended to address pituitary iron levels, we were unable to analyze certain structures (e.g., putamen, choroid plexus) previously shown to accumulate iron [6,9]. A prospective analysis of transfusion-dependent patients employing adapting QSM techniques to examine other brain regions would allow for an interesting comparison of methodologies within a single-center study.Conclusion
Quantitative MRI is an established non-invasive technique for the determination of iron excess of certain organs in chronically transfused patient groups. Calibration to true iron levels (e.g., determined via biopsy) and sequence optimization could extend its use to other potentially affected regions of the body, presenting a non-invasive continuous clinical monitoring tool. Determination of cerebral iron concentrations could allow for a deeper understanding of disease mechanism, potentially providing insight as to why patients afflicted with transfusion-dependent illness suffer cognitive deficits.Acknowledgements
No acknowledgement found.References
1. Ehlers KH, Levin AR, Markenson AL, et al. Longitudinal study of cardiac function in thalassemia major. Ann N Y Acad Sci. 1980;344:397–40.
2. Borgna-Pignatti C, Rugolotto S, De Stefano P, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89:1187–1193.
3. Wood JC. Impact of iron assessment by MRI. Hematology Am Soc Hematol Educ Program. 2011;443–50.
4. Hernando D, Levin YS, Sirlin CB, Reeder SB. Quantification of liver iron with MRI: state of the art and remaining challenges. J Magn Reson Imaging. 2014;40:1003-21.
5. Wood JC, Noetzl L, Hyderi A, Joukar M, Coates T, Mittelman S. Predicting pituitary iron and endocrine dysfunction. Ann N Y Acad Sci. 2010;1202:123-128.
6. Qiu D, Chan GC-F, Chu J, et al. MR quantitative susceptibility imaging for the evaluation of iron loading in the brains of patients with β-thalassemia major. AJNR Am J Neuroradiol. 2014;35(6):1085-1090.
7. Miao X, Choi S, Tamrazi B, et al. Increased brain iron deposition in patients with sickle cell disease: an MRI quantitative susceptibility mapping study. Blood. 2018;132(15):1618-1621.
8. Sedlacik J, Boelmans K, Löbel U, Holst B, Siemonsen S, Fiehler J. Reversible, irreversible and effective transverse relaxation rates in normal aging brain at 3T. Neuroimage. 2014;84:1032‐1041.
9. Akhlaghpoor S, Ghahari A, Morteza A, Khalilzadeh O, Shakourirad A, Alinaghizadeh MR. Quantitative T2* magnetic resonance imaging for evaluation of iron deposition in the brain of beta-thalassemia patients. Clin Neuroradiol. 2012;22(3):211–7.
10. Fung EB, Harmatz PR, Lee PD, et al. Increased prevalence of iron-overload associated endocrinopathy in thalassaemia versus sickle-cell disease. Br J Haematol. 2006;135(4):574-582.
11. Oudit GY, Trivieri MG, Khaper N, Liu PP, Backx PH. Role of L-type Ca2+ channels in iron transport and iron-overload cardiomyopathy. J Mol Med. 2006;84:349–364.
Figures