1571
Spatial Distribution Patterns of Secondary White Matter Tract Degeneration in Amyotrophic Lateral Sclerosis1Neuroradiology, Technische Universität Dresden, Dresden, Germany, 2Neurology, Technische Universität Dresden, Dresden, Germany, 3Neurology, Universitätsmedizin Rostock, Rostock, Germany, 4Alpert Medical School, Brown University, Providence, RI, United States
Synopsis
Amyotrophic lateral sclerosis (ALS) is a rapidly progressing neurodegenerative disease of primary cortical neuronal pathology and central nervous system multisystem involvement including related white matter. Based on previous findings of early myelination changes we aimed to investigate the spatial distribution of changes of markers of axonal (diffusion) and myelin (relaxation) integrity of the related motor corticospinal tract (CST). We found different pattern with minor, predominantly diffusion or predominantly relaxation, and extensive ubiquitary alteration. However, heterogeneous distribution of changes suggest a non-uniform secondary CST degeneration and limited prognostic value of ALS disease course.
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a rapidly progressing neurodegenerative disease of primary cortical neuronal pathology and central nervous system (CNS) multisystem involvement including related white matter (WM). Both aetiology and pathogenesis to date remain incompletely understood. The determinant progressive impairment of nearly all voluntary muscle function subsequently leads to respiratory failure and eventual death1. The clinical heterogeneity has lead to the assumption of a disease spectrum.We aimed to understand secondary WM tract degeneration spatial distribution in ALS by investigating potential quantitative markers of both axonal (diffusion) and myelin (relaxation) integrity using Diffusion Tensor Imaging (DTI) and the multi-component driven equilibrium single pulse observation of T1 and T2 (mcDESPOT). Based on previous findings of early myelination changes2 we hypothesized (1) non-uniform alterations of quantitative MRI measures within the corticospinal tract (CST), but (2) a cranio-caudal, i.e. centrifugal gradient in tract-based analysis, from degenerating cortex adjacent WM along the descending CST.
METHODS
Data was acquired using a SIEMENS Magnetom Verio 3T (Siemens Healthineers, Erlangen, Germany). DTI: EPI sequence, 2 two b/values (0/100), 64 diffusion directions, resolution of 2 mm^3. mcDESPOT: 1.7 mm^3 whole-brain FLASH (TR: 5.9ms, FA= [4, 5.3, 6.6, 8, 9.3, 12, 17.3, 24]°), trueFISP (TR 5.6ms; FA = [9.8, 13.1, 16.4, 19.7, 22.9, 29.5, 42.6, 59]°) and 1.7 mm^3 whole-brain inversion prepared FLASH (TR 5.7ms, FA = 5°, TI [450, 700] ms, PE 72). The quantitative parameters retrieved from diffusion data were mean diffusivity (MD), axial (AD) and radial diffusivity (RD) and fractional anisotropy (FA). Relaxation data provided T1 time and T2 time, myelin water fraction (MWF) and myelin water residence time (MRT)3.ALS patients (n=27) were scanned for the NiSALS (Neuroimaging Society in Amyotrophic Lateral Sclerosis) multi-site registry. Age matched healthy controls (n=21) were scanned as a comparative dataset. Ages and age ranges of both groups were tested for non-significant differences.
RESULTS
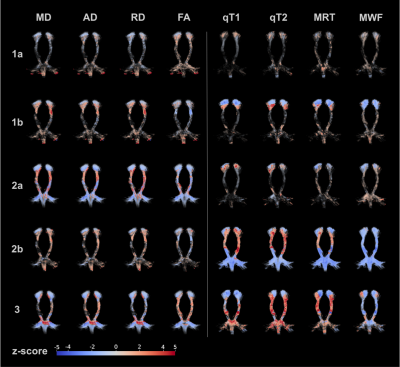
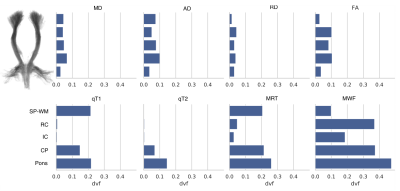
We found different pattern of individual diffusion and relaxation parameter alteration in CST: (1) minor or no alteration in both diffusion and relaxation parameters within CST (12/27 patients), within that group 6/12 with homogenously no alteration (1a) and 6/12 with some subcortical parameter deviation from normal, (2a) with predominantly diffusion (3/27) and (7/27) predominantly relaxation (2b) alteration, and, (3) homogenous alteration of diffusion and relaxation parameters (5/27) throughout CST (see Fig.1).Further tract-based voxel-wise analysis in CST subsegments revealed Deficient Volume Fractions (DVF)4 evenly distributed in diffusion metrics but inhomogeneous, craniocaudally increasing relaxation derived MRT and MWF (see Fig.2). Whereas MD, AD (p < 0.001) and RD (p < 0.05) demonstrated significant correlations with patients median age, just as qT1 (p < 0.05), qT2 (p < 0.001), MRT and MWF (p < 0.01), only qT1 (p < 0.05) revealed a significant correlation with ALS disease duration (Spearman’s rank correlation). Neither diffusion nor relaxation measures correlated significantly with the ALS-FRS (R) disease severity score.
DISCUSSION
Heterogeneous distribution of changes of markers of axonal (diffusion) and myelin (relaxation) integrity revealed a non-uniform secondary WM tract degeneration in primary neurodegenerative ALS. The diverse quantitative parameter deviation patterns may exhibit different subcohorts or the preservation and/or compensation capabilities in both the axonal and the myelin compartment.CONCLUSION
Based on these findings the prognostic value of secondary WM degeneration in ALS might be limited. However, the correlation of the found degeneration pattern and the disease progress remains subject to further investigation.Acknowledgements
No acknowledgement found.References
[1] Verde et al. Arch Ital Biol. 2017 Dec 1;155(4):118-130
[2] Kitzler et al. Proceedings Int Soc Mag Res Med. 2019:2779
[3] Deoni et al., Magn Reson Med. 2008; 6:1372-87
[4] Kitzler et al. NeuroImage. 2012;59(3):2670-7
Figures