1570
Disrupted grey matter network morphology in amyotrophic lateral sclerosis1Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Chengdu, China, 2University of Cincinnati, Cincinnati, OH, United States, 3Psychoradiology Research Unit of Chinese Academy of Medical Sciences (2018RU011), Chengdu, China
Synopsis
The present psychoradiological study demonstrated
significant alterations both in global and nodal topological properties of
single-subject brain morphological networks in a relatively large population of
patients with ALS relative to HCs. This was achieved by combining graph
theoretical analysis with a method of describing patterns of intercortical morphological
similarities in individual participants using structural MRI data. We found
that ALS showed a weaker small world organized brain network in global
properties and decreased nodal centralities mainly in prefrontal-limbic network
relative to HCs. Further, the nodal efficiency of right inferior frontal
gyrus was positively correlated to ALSFRS
in ALS group.
INTRODUCTION
Previous studies of amyotrophic lateral sclerosis (ALS) have reported functional alterations and white matter changes in localized brain regions 1 and connections 2. However, the topological organization of the brain morphological network in ALS was still unclear, we aimed to evaluate the topological organization of the whole brain network in ALS.METHODS
Study participants were consecutively recruited at the movement disorders outpatient clinic of West China Hospital of Sichuan University and this case-control study was approved by the local ethics committee, and written informed consent was obtained from all participants before enrollment. Eighty ALS patients and ninety-one demographically-matched healthy control (HC) subjects were both scanned to obtain a high-resolution 3D-T1 weighted structure image and we used the ALS function rating scale (ALSFRS) to assess the severity of ALS in patient group (Table 1). Statistical Parametric Mapping (SPM) software was used to prepossess the original data. The steps were as follows: (a) segment into grey matter (GM), white matter and CSF; (b) create the template to perform a nonlinear transformation; (c) normalize to the Montreal neurological institute (MNI) coordinate space, finally the GM image was resampled into 2 cubic millimeters of voxel and then spatially smoothed (Gaussian smoothing, 6 mm full width at half maximum). Then a network construction approach provided by Wang and Kong et al 3, 4 defined that parcellating the brain into different regions of interest in terms of automated anatomical labeling 90 template as node and a Kullback-Leibler divergence-based similarity measure was utilized to quantify morphological connectivity between two regions as edge, which was used to extract the single subject brain morphological network. Topological properties of brain networks including global (1. small world parameters: clustering coefficient (Cp), path length (Lp), normalized clustering coefficient (γ), normalized path length (λ), and small world index (σ) 5; 2. network efficiency 6: local network (Eloc) and global network efficiency (Eglob)) and nodal properties (nodal degree, nodal efficiency, and nodal betweenness 7) were constructed based on the morphological similarity of GM across regions with graph-theory approach by GRETNA. Nonparametric permutation test was used to determine whether there were significant differences in the area under the curve (AUC) of all of the network metrics 8 between HC and ALS. And we used network-based statistics (NBS) to identify the region pairs with between-group differences. After significant between-group differences had been identified in the network metrics, partial correlations using age and gender as covariates were performed to evaluate relationships between network metrics and clinical scores.RESULTS
Compared with HC, ALS exhibited decreased global efficiency (Eglob) (p=0.002), clustering coefficient (Cp) (p=0.032), and small world index (p=0.002) (Figure 1) as well as abnormal centrality in nodes mainly decreased in prefrontal-limbic circuit, including bilateral superior/inferior frontal gyrus, right precentral gyrus and middle frontal gyrus, right superior/middle temporal gyrus and increased in lenticular nucleus, left thalamus. Network alterations of brain regions showing between-group differences of nodal properties, we identified significantly decreased connectivity alterations within networks composing 11 nodes and 13 edges in ALS group (Table 2 and Figure 2). And ALSFRS was positively correlated with the nodal efficiency of right inferior frontal gyrus in ALS group (r=0.227, p=0.045) (Figure 3).DISCUSSION
The significantly decreased Eglob, Cp and small world index in ALS compared with HC showed a weaker small world configuration, which was also reported in bipolar disorder 9, schizophrenia 10 and posttraumatic stress disorder 11, whose connectivity network have been identified closer to weaker small world. Due to the small world model reflects an optimal balance between the segregation (clustering coefficient (Cp) or local efficiency (Eloc)) and integration (path length (Lp), and global efficiency (Eglob)), thus our results would indicate a disturbance of the normal balance in the brain morphological networks of ALS patients. Besides, in nodal level, Zhou et al.12 have reported that abnormal regions mainly focused on the limbic system in ALS, and Agosta et al.13 have also reported that ALS patients in a series of brain regions broadly related to emotional functions have reported both reduced and enhanced responses, those were consistent with our result, indicating that for ALS it was easier to trigger a transfer of the corresponding emotion. In addition, confirming the previous findings, brain network of patients had reduced levels of anatomic connectivity of pairs of brain regions, we can speculate that this reduction might be associated with the reduced structural capacity to integrate information among different regions of the brain, resulting in more functionally isolated subsystems. Another result showed that the nodal efficiency of right inferior frontal gyrus of topological organization in the morphological network of ALS patients was significantly correlated with ALSFRS. Previous neuropathological and structural MRI studies have shown consistently that ALS is associated with an extensive involvement of the frontal regions 14, 15, it was consistent with that in our study. So, we can speculate that the higher efficiency of right inferior frontal gyrus value and the more serious ALS symptom.CONCLUSIONS
Our findings suggest that ALS patients showed significant structural topological alternations and abnormal connections when compared with HCs. Furthermore, the present psychoradiological findings could help to clarify the pathogenesis of ALS and could be potential biomarkers of brain abnormalities.Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant Nos.81621003, 81761128023, 81220108013, 81227002, 81030027), the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, grant IRT16R52) of China, the Changjiang Scholar Professorship Award (Award No.T2014190) of China, and the CMB Distinguished a Professorship Award (Award No.F510000/G16916411) administered by the Institute of International Education.References
1. Dimond, D., et al., White matter structural network abnormalities underlie executive dysfunction in amyotrophic lateral sclerosis. Hum Brain Mapp, 2017. 38(3): p. 1249-1268.
2. Ma, X., et al., Altered cortical hubs in functional brain networks in amyotrophic lateral sclerosis. Neurol Sci, 2015. 36(11): p. 2097-104.
3. Wang, H., et al., Single-subject morphological brain networks: connectivity mapping, topological characterization and test-retest reliability. Brain Behav, 2016. 6(4): p. e00448.
4. Kong, X.Z., et al., Measuring individual morphological relationship of cortical regions. J Neurosci Methods, 2014. 237: p. 103-7.
5. Watts, D.J. and S.H. Strogatz, Collective dynamics of 'small-world' networks. Nature, 1998. 393(6684): p. 440-2.
6. Latora, V. and M. Marchiori, Efficient behavior of small-world networks. Phys Rev Lett, 2001. 87(19): p. 198701.
7. Achard, S. and E. Bullmore, Efficiency and cost of economical brain functional networks. PLoS Comput Biol, 2007. 3(2): p. e17.
8. Zhang, J., et al., Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol Psychiatry, 2011. 70(4): p. 334-42.
9. Collin, G., et al., Brain network analysis reveals affected connectome structure in bipolar I disorder. Hum Brain Mapp, 2016. 37(1): p. 122-34.
10. van den Heuvel, M.P., et al., Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry, 2013. 70(8): p. 783-92.
11. Mueller, S.G., et al., Evidence for disrupted gray matter structural connectivity in posttraumatic stress disorder. Psychiatry Res, 2015. 234(2): p. 194-201.
12. Zhou, C., et al., Altered Brain Network in Amyotrophic Lateral Sclerosis: A Resting Graph Theory-Based Network Study at Voxel-Wise Level. Front Neurosci, 2016. 10: p. 204.
13. Agosta, F., et al., Divergent brain network connectivity in amyotrophic lateral sclerosis. Neurobiol Aging, 2013. 34(2): p. 419-27.
14. Agosta, F., et al., The present and the future of neuroimaging in amyotrophic lateral sclerosis. AJNR Am J Neuroradiol, 2010. 31(10): p. 1769-77.
15. Douaud, G., et al., Integration of structural and functional magnetic resonance imaging in amyotrophic lateral sclerosis. Brain, 2011. 134(Pt 12): p. 3470-9.
Figures
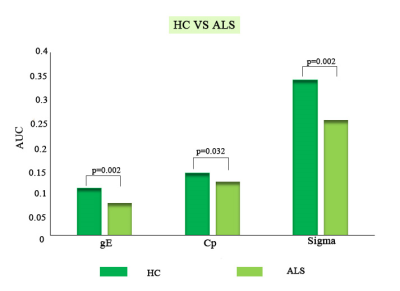
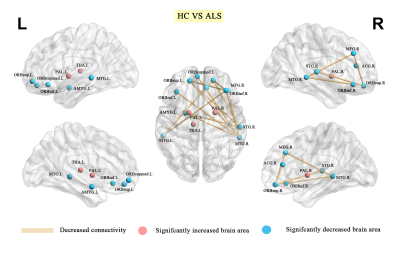

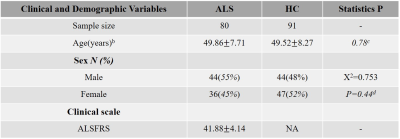
Table 1. Demographic data and clinical characteristics of study participants a.
HC: healthy control; ALS: Amyotrophic lateral sclerosis. ALSFRS: Amyotrophic lateral sclerosis functional rating scale.
a Data are presented as means ± standard deviations. No significant differences were identified between ALS and HC in age and gender.
b Age was defined at the time of MRI scanning.
c P value was obtained by two-tailed two-sample t test, P < 0.05.
d P value was obtained by two-tailed Pearson Chi-square test, P < 0.05.

Table 2. Regions with significant alterations in ALS patients in comparison with HCs.
HC: healthy control; ALS: Amyotrophic lateral sclerosis. Regions are listed above if there were significant between-group differences in at three nodal centrality parameters.
1. The Benjamini–Hochberg false discovery rate correction was applied to correct for multiple group comparisons of brain regions and the P value threshold is 0.05;
2. All the brain regions are from AAL (automated anatomical labeling);
3. Abbreviation: R: right, L: left.