1567
Identification of amyotrophic lateral sclerosis based on diffusion tensor imaging and support vector machine1Fujian Medical University Union Hospital, Fuzhou, China, 2SIEMENS Healthcare, Shanghai, China
Synopsis
White matter (WM) impairments have been well documented in amyotrophic lateral sclerosis (ALS). This study tested the potential of diffusion measurements in WM for identifying ALS based on the support vector machine (SVM). In the optimized SVM model, the FA values from the motor areas (including the bilateral precentral gyrus and the corticospinal tract) and extra-motor areas (including right postcentral gyrus, left superior/inferior longitudinal fasciculus) contributed mostly to classification. Our study suggests the feasibility of ALS diagnosis based on SVM analysis and diffusion measurements of WM. Future study with larger cohort is needed to validate the generality of our results.
Introduction
As a cryptogenetic and fatal neurodegenerative disorder, amyotrophic lateral sclerosis (ALS) often occurs in adults. This is a heterogeneous disease, with difficulty in early diagnosis. From the onset of symptoms to death, the average survival time is 3-5 years [1], and Riluzole can only prolong the survival time of 2-3 months [2]. Therefore, early diagnosis of ALS and detection of its pathogenesis are particularly important.Diffusion tensor imaging (DTI) plays a key role in investigating neuropathology of neurodegenerative disorders. The major parameter of white matter (WM) fibers anisotropy is FA (a symbol of white matter integrity). Several DTI studies have revealed that ALS patients showed decreased FA in the corticospinal tract (CST) and extra-motor areas [3]. Actually, the decreased FA in the CST and corpus callosum is a promising biomarker candidate to diagnose and evaluate ALS [4].
Support vector machine (SVM), a kind of machine learning algorithm, has been widely used to investigate the discriminative brain map for patients with psychiatric and neuropathic disorders. A prior resting-state functional magnetic resonance imaging study has used SVM to identify ALS based on the functional connectivity measurement in brain networks [5]. However, to our knowledge, there is no research using SVM to discriminate ALS patients based on DTI measurement. Thus, the objective of this study is to test the potential of diffusion measurements in WM for identifying ALS based on the SVM learning method.
Methods
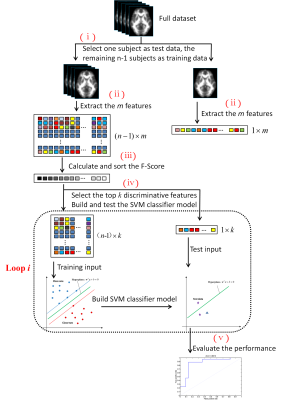
Voxel-wise fractional anisotropy (FA) values of the diffusion tensor images (DTI) were extracted from 22 ALS patients and 26 healthy controls and served as discrimination features. The disease severity of ALS was assessed based on the revised ALS Functional Rating Scale (ALSFRS-R). The feature ranking and selection were based on Fisher score. A linear kernel SVM algorithm was applied to build the classification model, from which the classification performance was evaluated. To promote the classifier generalization ability, a leave-one-out cross-validation (LOOCV) strategy was then adopted.Results
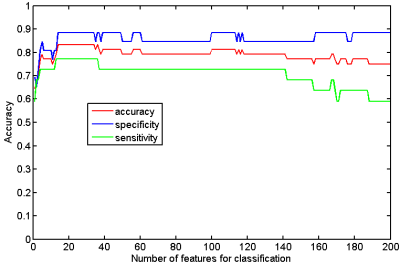
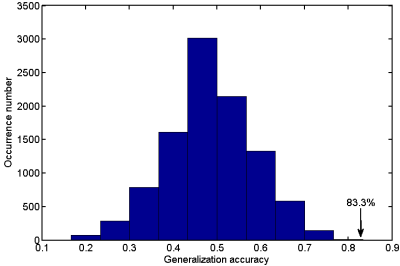
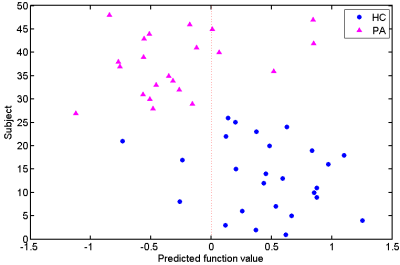
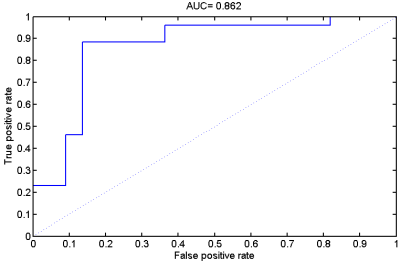
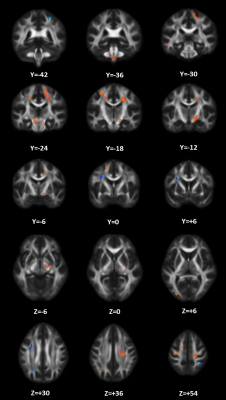
When taking the 2400~3400 ranked features as the optimal features, we achieved the high classification accuracy of 83.33% (88.46% for sensitivity, 77.27% for specificity, P=0.0001), with the area under receiver operating characteristic curve of 0.862. The predicted function value was positively correlated with patients’ ALSFRS-R score (r=0.493, P=0.020). In the optimized SVM model, the FA values from the following regions contributed mostly to classification: bilateral corona radiate and precentral gyrus, right postcentral gyrus, right posterior limb of internal capsule, left superior frontal gyrus, left angular gyrus, left middle temporal gyrus, left middle occipital gyrus, bilateral midbrain, bilateral pons and medulla, left frontal lobe, left inferior parietal lobule, and right superior parietal lobule.Discussion
In the present study, we combined DTI with SVM to classify ALS patients and healthy controls. The high classification accuracy of 83.33% can be obtained in the optimized SVM model. The FA values from both motor and extra-motor areas contributed to classification, which indicated that ALS is a multiple system neurodegenerative disease. Moreover, the predicted function value correlated with ALS disease severity (indexed by ALSFRS-R score). The further ROC analysis and permutation statistics further validate the reliability of our SVM classifier.Consistent with previous studies [6; 7], we found that the regions with decreased FA involved the bilateral precentral gyrus and the CST (such as bilateral corona radiate, right posterior of internal capsule, bilateral midbrain, bilateral pons and medulla). The precentral gyrus is part of the primary motor cortex (PMC), degenerative alterations of the PMC in ALS include significantly decreased Betz cells and cortical thinning [8; 9]. The CST is the area of cortical control of spinal cord activity, connecting the neurons in motor cortex and spinal cord [10]. Degeneration of the CST is a hallmark feature of ALS [11]. To sum up, the damage of these motor regions leads to motor neuron dysfunction (e.g. muscle weakness, loss of voluntary control ) in ALS patients [12].
The regions with decreased FA also involved several the extra-motor areas, such as right postcentral gyrus, left superior longitudinal fasciculus (SLF) (including left superior frontal gyrus, left angular gyrus and left middle temporal gyrus) and left inferior longitudinal fasciculus (ILF) (including left middle temporal gyrus and left middle occipital gyrus), which consistent with previous studies [13; 14]. For example, it has been demonstrated that the significant cortical thinning of the postcentral gyrus (namely primary somatosensory cortex [15]) occurred in ALS. Also, the SLF, connecting frontal, parietal and temporal lobes and playing a key role in language function [16], is disrupted in ALS [17; 18]. Meanwhile, the damage to the left ILF (this fiber primary associated with visual processing, language/semantic function, and the regulation of emotion [19]), has been revealed in ALS patients [20]. Therefore, the damage of these extra-motor regions (reflected by decreased FA) may be associated with the non-motion dysfunctions in ALS, such as sensory deficits, language dysfunction, behavioral and psychiatric abnormalities [21; 22].
Conclusions
Our results suggest the feasibility of ALS diagnosis based on SVM analysis and diffusion measurements of WM. Future study with larger cohort is needed to validate the generality of our results.Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81501450), Fujian Provincial Science Fund for Distinguished Young Scholars (No. 2018J06023), Fujian Provincial Program for Distinguished Young Scholars (No. 2017B023), and Fujian Provincial Health Commission Project for Scientific Research Talents (No. 2018-ZQN-28).References
[1] Hardiman O, van den Berg LH, Kiernan MC (2011) Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat Rev Neurol 7:639-649
[2] Miller RG, Mitchell JD, Moore DH (2012) Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database of Systematic Reviews. 10.1002/14651858.CD001447.pub3
[3] Zhang F, Chen G, He M et al (2018) Altered white matter microarchitecture in amyotrophic lateral sclerosis: A voxel-based meta-analysis of diffusion tensor imaging. Neuroimage Clin 19:122-129
[4] Mazon M, Vazquez Costa JF, Ten-Esteve A, Marti-Bonmati L (2018) Imaging Biomarkers for the Diagnosis and Prognosis of Neurodegenerative Diseases. The Example of Amyotrophic Lateral Sclerosis. Front Neurosci 12:784
[5] Welsh RC, Jelsone-Swain LM, Foerster BR (2013) The utility of independent component analysis and machine learning in the identification of the amyotrophic lateral sclerosis diseased brain. Front Hum Neurosci 7:251
[6] Sage CA, Van Hecke W, Peeters R et al (2009) Quantitative diffusion tensor imaging in amyotrophic lateral sclerosis: revisited. Hum Brain Mapp 30:3657-3675
[7] Agosta F, Pagani E, Petrolini M et al (2010) Assessment of white matter tract damage in patients with amyotrophic lateral sclerosis: a diffusion tensor MR imaging tractography study. AJNR Am J Neuroradiol 31:1457-1461
[8] Mochizuki Y, Mizutani T, Shimizu T, Kawata A (2011) Proportional neuronal loss between the primary motor and sensory cortex in amyotrophic lateral sclerosis. Neurosci Lett 503:73-75
[9] Verstraete E, Veldink JH, Hendrikse J, Schelhaas HJ, van den Heuvel MP, van den Berg LH (2012) Structural MRI reveals cortical thinning in amyotrophic lateral sclerosis. Journal of Neurology, Neurosurgery & Psychiatry 83:383-388
[10] Welniarz Q, Dusart I, Roze E (2017) The corticospinal tract: Evolution, development, and human disorders. Dev Neurobiol 77:810-829
[11] Sarica A, Cerasa A, Valentino P et al (2017) The corticospinal tract profile in amyotrophic lateral sclerosis. Hum Brain Mapp 38:727-739
[12] Esquenazi A, Mayer NH, Elia AE, Albanese A (2009) Botulinum toxin for the management of adult patients with upper motor neuron syndrome. Toxicon 54:634-638
[13] Trojsi F, Corbo D, Caiazzo G et al (2013) Motor and extramotor neurodegeneration in amyotrophic lateral sclerosis: a 3T high angular resolution diffusion imaging (HARDI) study. Amyotroph Lateral Scler Frontotemporal Degener 14:553-561
[14] Crespi C, Cerami C, Dodich A et al (2014) Microstructural white matter correlates of emotion recognition impairment in Amyotrophic Lateral Sclerosis. Cortex 53:1-8
[15] Kaas JH, Nelson RJ, Sur M, Lin CS, Merzenich MM (1979) Multiple representations of the body within the primary somatosensory cortex of primates. Science 204:521-523
[16] Conner AK, Briggs RG, Rahimi M et al (2018) A Connectomic Atlas of the Human Cerebrum-Chapter 10: Tractographic Description of the Superior Longitudinal Fasciculus. Oper Neurosurg (Hagerstown) 15:S407-S422
[17] Abrahams S, Goldstein LH, Suckling J et al (2005) Frontotemporal white matter changes in amyotrophic lateral sclerosis. J Neurol 252:321-331
[18] Zhang J, Yin X, Zhao L et al (2014) Regional alterations in cortical thickness and white matter integrity in amyotrophic lateral sclerosis. Journal of Neurology 261:412-421
[19] Sali G, Briggs RG, Conner AK et al (2018) A Connectomic Atlas of the Human Cerebrum-Chapter 11: Tractographic Description of the Inferior Longitudinal Fasciculus. Oper Neurosurg (Hagerstown) 15:S423-S428
[20] Westeneng HJ, Walhout R, Straathof M et al (2016) Widespread structural brain involvement in ALS is not limited to the C9orf72 repeat expansion. J Neurol Neurosurg Psychiatry 87:1354-1360
[21] Vucic S (2017) Sensory and autonomic nervous system dysfunction in amyotrophic lateral sclerosis. Neuropathology and Applied Neurobiology 43:99-101
[22] McCombe PA, Wray NR, Henderson RD (2017) Extra-motor abnormalities in amyotrophic lateral sclerosis: another layer of heterogeneity. Expert Rev Neurother 17:561-577
Figures