Thomas Welton1, Sarah C Hellewell1, Michel Tchan2, and Stuart M Grieve1
1University of Sydney, Sydney, Australia, 2Department of Genetic Medicine, Westmead Hospital, Sydney, Australia
Synopsis
Diffusion
kurtosis MRI (DKI) was used to measure changes in white matter structure in 20 patients
with phenylketonuria and 43 controls. We found significant differences primarily
in the periventricular parietal white matter in various DKI metrics. These
scores were related to phenylalanine levels measured in the 3 years prior to
MRI and with Scheltens score, reflecting white matter hyperintensities. DKI may
be sensitive to pathology invisible to clinical MRI, and we propose a simple metric
in the parietal lobe which robustly captures this effect.
Introduction
Phenylketonuria
(PKU) is an autosomal recessive disorder of amino acid metabolism in which untreated,
accumulation of phenylalanine (Phe) metabolites leads to progressive
demyelination [1]. Phe levels are used to monitor
PKU status, as opposed to any neuronal- or cognitive-based measure, and is
treated with a low-protein diet. Non-diet-compliant patients often develop
cognitive deficits and periventricular white matter (WM) hyperintensities [2]. Diffuse WM pathology may also be
present [3] but may not be measurable with
conventional imaging. Diffusion kurtosis imaging (DKI) is an extension of
diffusion tensor imaging which uses kurtosis of the water diffusion probability
distribution function to account for the non-Gaussianity of diffusion in
biological tissue, and can detect subtle changes in brain parenchymal structure.
DKI may, therefore, be sensitive to WM pathology in PKU [4]. We
investigated the feasibility of DKI as a quantitative biomarker for
longitudinal monitoring in clinically well-characterized adults with PKU, with
the hypothesis that periventricular WM may show the largest abnormalities,
compared to deep and peri-cortical WM.Methods
Our cohort of 20
patients was diagnosed with PKU at birth and variably adhered to a low-protein
diet. A control cohort of 43 healthy individuals was matched for gender and age.
Plasma Phe concentrations were gathered from clinical records and a computerized
neurocognitive battery was performed using WebNeuro [5].
Multi-band DKI
data were acquired over 3 shells with b-values of 700, 1000, and 2800 mm/s2,
66 slices, 140 unique gradient directions, TR=4323ms, TE=91.80ms, flip
angle=90°, matrix=128x128, voxel dimensions=2mm isotropic and one reverse
phase-encoded b=0 volume. DKI data were processed with Diffusion Kurtosis
Estimator [6] to create axial, radial, mean and
fractional anisotropy diffusion and kurtosis images. Personalised masks were
generated from T1-weighted images of the lobes, periventricular, deep, and peri-cortical
WM and co-registered to the DKI data. Scheltens score was measured in each
patient by a clinical neuroradiologist to quantify WM hyperintensities. We
tested for group differences in WM characteristics across our masks. Post hoc
analyses were conducted following key findings by correlation with Scheltens
score and Phe level in the 3 years prior to imaging.Results
The groups were
matched for age (PKU, 35.2±11.5 years; control, 32.8±13.3 years; t-test, t=-0.69,
p=0.47) and gender (PKU, 50% female; control, 46% female; χ2=0.067,
p=0.80). The PKU group had significantly less cerebral WM (t=2.25, p=0.028), less
total intracranial volume (t=2.99, p=0.004), and less CSF (t=2.28, p=0.026).
Based on radiological
impression, we focussed our analyses on the parietal lobe. Axial diffusivity in
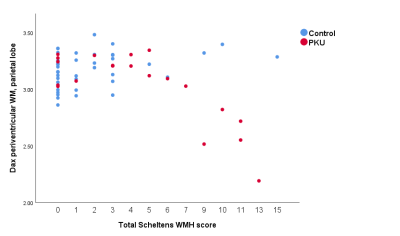
the periventricular WM of the parietal lobe clearly differentiated the groups (t=2.55,
p=0.013). When comparing this measure across Scheltens scores, controls
remained stable, while PKU patients’ parietal periventricular axial diffusivity
measure decreased at high Scheltens scores (Figure 1). This measure was highly
correlated with mean Phe level across the 3 years prior to imaging (r=-0.607, p=0.005),
but not lifetime mean Phe level. At Scheltens scores above 6, the groups were
fully differentiated.
In the
periventricular parietal WM, radial and axial kurtosis were also significantly
different between groups (t=0.553, p=0.011; t=-9.59, p=0.021), as well as
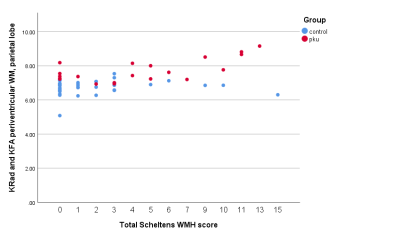
kurtosis FA (t=2.53, p=0.014). Based on these findings and inspection of
scatter plots, we formed a composite measure as the ratio between radial
kurtosis and kurtosis FA, measured in the periventricular parietal WM (Figure 2) which
strongly differentiated the groups (t=-7.17, p<0.001) and strongly
correlated with total Scheltens score (r=0.496, p<0.001).Conclusions
PKU may result in
progressive WM changes which are invisible to conventional MRI. Importantly, these
changes can occur even in diet-compliant patients, indicating a need for more
sensitive, patient-specific monitoring. Our DKI findings suggest highlight the potential
for a role of these metrics as future quantitative biomarkers of WM pathology
in PKU. Acknowledgements
No acknowledgement found.References
1. Williams, R.A., C.D.S.
Mamotte, and J.R. Burnett, Phenylketonuria:
an inborn error of phenylalanine metabolism. The Clinical biochemist.
Reviews, 2008. 29(1): p. 31-41.
2. Leuzzi,
V., et al., The pathogenesis of the white
matter abnormalities in phenylketonuria. A multimodal 3.0 tesla MRI and
magnetic resonance spectroscopy (1H MRS) study. Journal of Inherited
Metabolic Disease, 2007. 30(2): p.
209-216.
3. Phillips,
M.D., et al., Diffusion-Weighted Imaging
of White Matter Abnormalities in Patients with Phenylketonuria. American
Journal of Neuroradiology, 2001. 22:
p. 1583-1586.
4. Arab,
A., et al., Principles of diffusion
kurtosis imaging and its role in early diagnosis of neurodegenerative
disorders. Brain Res Bull, 2018. 139:
p. 91-98.
5. Silverstein,
S.M., et al., Development and validation
of a World-Wide-Web-based neurocognitive assessment battery: WebNeuro.
Behav Res Methods, 2007. 39(4): p.
940-9.
6. Tabesh,
A., et al., Estimation of tensors and
tensor-derived measures in diffusional kurtosis imaging. Magn Reson Med,
2011. 65(3): p. 823-36.