1557
Neuro-inflammation is associated with WMH burden at baseline and predicts longitudinal cognitive decline in cerebral small vessel disease1Mallinckrodt Institute of Radiology, Washington University School of Medicine, St. Louis, MO, United States, 2Department of Neurology, Washington University School of Medicine, St. Louis, MO, United States, 3Department of Neurological Surgery, Washington University School of Medicine, St. Louis, MO, United States
Synopsis
Neuro-inflammation has been suggested as an important pathogenesis factor for cerebral small vessel disease, but direct evidence in human is lacking. In this study, we found that neuro-inflammation, measured by 11C-PK11195 uptake, was associated with white matter hyperintensities burden at baseline. More importantly, it predicted cognitive decline in a longitudinal follow-up study.
Introduction
Fifty million people are affected by dementia worldwide1. Vascular contributions to cognitive impairment and dementia (VCID) is the 2nd leading cause of dementia after Alzheimer’s disease (AD). White matter hyperintensities (WMH) is a major structural endpoint of cerebral small vessel disease (CSVD), a major cause of VCID. The pathogenesis underlying CSVD is not well understood. Several systemic inflammatory markers were detected in blood and CSF2. More recently, increased and persistent systemic inflammation (measured by CRP) in midlife was found to promote late-life white matter structural injury3, 4. Activated microglia was found in post-mortem histological of CSVD patients5. Therefore, neuro-inflammation has been suggested as an important pathogenesis factor for CSVD. However, postmortem studies only reflect neuro-inflammation in the late stages of disease and direct evidence of in vivo neuro-inflammation as an early biomarker from humans is lacking. Expression of translocator protein (TSPO) is strongly upregulated upon microglial activation due to neuro-inflammation. Therefore, PET tracers, such as 11C-PK11195, that bind to TSPO allows imaging neuro-inflammation in vivo5.In this study, we evaluated whether neuro-inflammation, measured by 11C-PK11195 uptake, is associated with WMH burden at time of imaging (baseline). Moreover, we aimed to examine whether neuro-inflammation predicts cognitive decline in a longitudinal follow-up study. Due to a high prevalence of mixed CSVD and AD pathology in patients, we also examined whether neuro-inflammation is associated with amyloid deposition, a hallmark pathological feature of AD.
Methods
Twenty-four elderly subjects (Age: 78 [64.75 83] (median [IQR]); Education level: 16 [14 18] (median [IQR]) years; 14 female) were recruited. 11C-PK11195 PET imaging were acquired from each subject. Two 11C-PiB PET scans were acquired. The 1st scan was obtained 15.5 [12.5 20] (median [IQR]) months before the 11C-PK11195 PET from all subjects, and the 2nd scan was obtained 33 [27.75 80.75] months (median [IQR]) after the 11C-PK11195 PET from 18 subjects. MR FLAIR and T1 MPRAGE images were acquired 6 [2 15.5] (median [IQR]) months before 11C-PK11195 PET from 19 subjects. Cognitive data were collected using Knight standard ADRC battery. The baseline cognitive data were collected 56.5 [-42.5 210.25] (median [IQR]) days after 11C-PK11195 PET and these patients were followed up for 6.5 [4.25 11] (median [IQR]) years.T1 MPRAGE images were segmented using Freesurfer software. 11C-PK11195 PET standardized uptake value (SUV) maps were computed. Standardized uptake value ratio (SUVR) maps were computed with median cerebellum gray matter SUV as reference. Mean cortical binding potential (MCBP) of 11C-PiB were calculated using PET Unified pipeline (PUP) 6. WMH lesions were delineated manually by a board-certified vascular neurologist on FLAIR images. To account for brain volume variation across subjects, relative WMH volume (rVWMH) was computed as a ratio of WMH lesion volume (VWMH) to brain tissue volume. All MR and PET images and maps were aligned to International Consortium of Brain Mapping (ICBM) brain atlas. Three standardized composite z-scores, zglobal, zspeed and zmemory, were calculated to assess global, processing speed, and memory cognitive functions respectively.
Results
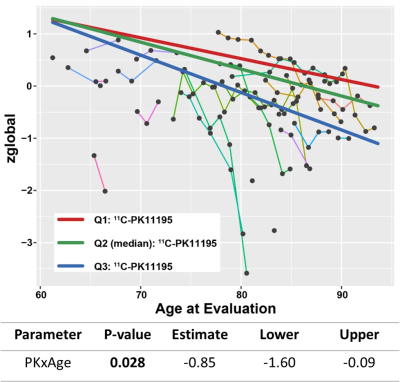
All patients had various levels of WMH lesion burden with a median [IQR] VWMH of 8.3 [3.6 24.2] cm3, corresponding to rVWMH of 1.05% [0.35% 2.81%] (median [IQR]). These patients had relatively normal cognitive functions at the time of imaging. Only 7 out of 24 subjects had a baseline clinical dementia rate (CDR) greater than 0 (CDR=0.5: n=5, CDR=1: n=2). A multivariate linear regression model controlling for difference in acquisition date of 11C-PK11195 PET and FLAIR showed that WMH lesion burden at baseline was significantly associated with gray matter 11C-PK11195 uptake (p=0.011) and age (p=0.028). However, 11C-PiB MCBP was not significantly associated with either WMH lesion burden or 11C-PK11195 uptake, suggesting that neuro-inflammation is independent of amyloid deposition.Longitudinal analysis of cognitive change over time using regional 11C-PK11195 SUVR and 11C-PiB MCBP was performed using linear mixed-effects regression with random subject effects and time delay between the acquisition of PET data and baseline cognitive tests. Interaction between PET tracer and age at cognitive evaluation was assessed as primary outcome. We found that elevated gray matter 11C-PK11195 uptake and old age together predicted global cognitive decline (β=-0.85/year, p=0.028) (Figure 1). We also found that elevated 11C-PiB MCBP of the 2nd 11C-PiB scan and old age predicted both global cognitive decline (β=-0.1083/year, p<0.0001) and memory impairment (β=-0.0819/year, p<0.0001), while the 11C-PiB MCBP of the 1st 11C-PiB did not predict any cognitive decline.
Discussion
11C-PK11195 uptake was associated with WMH lesion burden at baseline. More importantly, elevated 11C-PK11195 and 11C-PiB MCBP were not associated with each other and both predicted cognitive decline in longitudinal follow-up, suggesting neuro-inflammation and amyloid deposition may be independent pathogenesis factors for AD and/or CSVD.Conclusion
We found that 11C-PK11195 uptake was significantly associated with baseline WMH lesion burden, but not with 11C-PiB MCBP. A longitudinal follow-up study showed elevated 11C-PK11195 uptake in gray matter preceded cognitive impairment and neuro-inflammation and old age together predicted cognitive decline. Our results suggest that neuro-inflammation is an independent early biomarkers for neurodegenerative disease.Acknowledgements
No acknowledgement found.References
1. Gorelick, Philip B., et al. "Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association." Stroke 42.9 (2011): 2672-2713.
2. Rosenberg, Gary A. "Extracellular matrix inflammation in vascular cognitive impairment and dementia." Clinical Science 131.6 (2017): 425-437.
3. Walker, Keenan A., et al. "Midlife systemic inflammation, late-life white matter integrity, and cerebral small vessel disease: the atherosclerosis risk in communities study." Stroke 48.12 (2017): 3196-3202.
4. Walker, Keenan A., et al. "The association of mid-to late-life systemic inflammation with white matter structure in older adults: The Atherosclerosis Risk in Communities Study." Neurobiology of aging 68 (2018): 26-33.
5. Evans, Nicholas R., et al. "PET imaging of the neurovascular interface in cerebrovascular disease." Nature Reviews Neurology 13.11 (2017): 676-688.
6. Su, Yi, et al. "Quantitative analysis of PiB-PET with freesurfer ROIs." PloS one 8.11 (2013): e73377.