1554
Diffusion kurtosis imaging for characterizing the microstructural changes of hippocampus in mild cognitive impairment patients with cerebral small vascular disease.1Department of Neurology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China, 2Department of Radiology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China, 3MR Scientific Marketing, Diagnosis Imaging, Siemens Healthcare Ltd, Beijing, China
Synopsis
We investigated the early microstructural alterations in hippocampus in MCI patients with cSVD by DKI. Our study found that MCI patients with cSVD show more seriously white matter hyperintensity, and showed significantly increased MD and RD values, and decreased MK, AK, RK, FA and KFA values in left hippocampus. In left hippocampus, values of FA, MK, RK, and KFA showed significantly positive correlations with MoCA score, while MD and RD values were negatively correlated with MoCA score. This may be due to the loss of neuron cell bodies, synapses and dendrites. DKI technique may be feasible to probe the microstructural changes of hippocampus in MCI patients with cSVD.
Background and Purpose
Mild cognitive impairment (MCI) is common in senior adults, and its prevalence increases with age and limited educational level. A previous study has revealed a 2.2-fold higher volume loss in the hippocampus in MCI patients [1]. In recent years many studies have focused on the diagnostic applications of diffusion tensor imaging (DTI) [2-6]. However, only few DKI studies are available to better understand the changes in hippocampus in patients with MCI. This study analyzed the kurtosis parameters of patients with MCI, and found the early microstructural alterations in hippocampus in patients with MCI.Material and Methods
A total of 82 patients with cSVD (especially with white matter hyperintensity, WMH) confirmed by conventional MRI scans (including MRA) were enrolled. The Montreal cognitive assessment score (MoCA) scale was used to assess the overall cognitive function [8]. According to the presence or absence of MCI, 82 patients were divided into MCI group (n=48) and non-MCI group (n=34). Data were collected on a 3T MAGNETOM Skyra scanner (Siemens Healthcare, Erlangen, Germany) with a 20-channel head coil. T1-weighted images (T1WI) were scanned using a 3D magnetization-prepared rapid acquisition gradient echo (MPRAGE), with the following sequence parameters: repetition time (TR) = 2300ms, inversion time (TI) = 900ms, echo time (TE) = 89ms, flip angle (FA) = 8°, field-of-view (FOV) = 240mm×240mm,voxel size = 0.9 mm isotropic, the parallel acceleration factor (PAT) = 2, and the acquisition time is 5 min 21 sec. The diffusion weighted imaging was performed using a spin-echo echo planar imaging (SE-EPI), and was scanned in two blocks: (1) The parameters of the first block is TR = 7700ms, TE = 89ms, imaging matrix = 74×74, FOV = 222mm×222mm, number of slices = 50, slice thickness = 3mm, b=0, 1000, 2000 s/mm2, 1 average, 30 gradient directions, PAT=2, and the acquisition time is 8 min 14 sec; (2) The parameter of the second block is the same as the first one except only b=0 s/mm2 was used, 9 average, acquisition time is 1 min 34 sec. The total scan time of diffusion imaging is 9 min 48 sec. The scanned diffusion-weighted images were first transformed to NIFTI file format using dcm2nii tool, and then imported to the diffusional kurtosis estimator (DKE) to generate DKI parameter maps. We use the Anatomical Automatic Labeling (AAL) template to automatically segment hippocampus with an atlas-based registration [7]. The regions of interests (ROIs) of hippocampus were selected by label indices of 37 and 38 according to AAL template. Specifically, the T1W images acquired by MP-RAGE were supplied to SPM12 toolbox [9]. The AAL template was nonlinearly registered to T1W images, and the AAL labels were transformed to the T1W image space using the generated wrapping field and transformation matrix. The DWI images (b=0 s/mm2) were rigidly aligned to the T1WI space, and the corresponding transformed matrix was applied onto the DKI parameter maps. Then, the average values of mean diffusion (MD), axial diffusion (AD), radial diffusion (RD), fractional anisotropy (FA), mean kurtosis (MK), axial kurtosis (AK), radial kurtosis (RK) and kurtosis fractional anisotropy (KFA) in hippocampus were automatically calculated using MATLAB (2017a, The MathWorks, Inc., Natick, MA), and were compared between two groups. The correlations were analyzed between DKI parameters and MoCA score. P<0.05 was considered statistically significant.Results
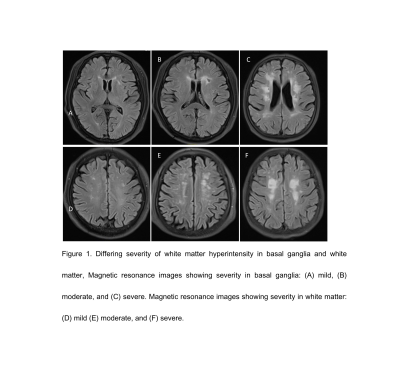
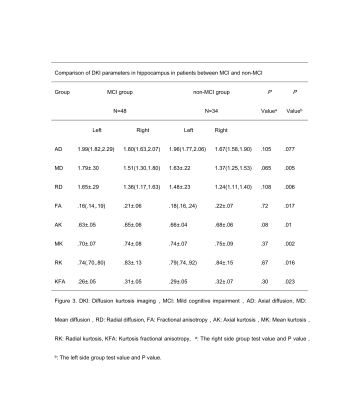
Compared to non-MCI group, MCI group had more severity white matter hyperintensity (WMH) patients (p=0.01, 95%CI 0.28-0.61, see in figure 1 and Table 2). MCI group showed significantly increased MD and RD (P=0.005, P=0.006), and significantly decreased MK, AK, RK, FA and KFA values in left hippocampus (P=0.002, 0.01, 0.016, 0.017, 0.023 respectively, Table 3). In left hippocampus, FA, MK, RK, and KFA showed significantly positive correlations with MoCA score (r=0.374, 0.37, 0.392, 0.242, respectively, P<0.05), while MD, and RD were negatively correlated with MoCA score (r=-0.227,-0.255 respectively, P<0.05, Figure 4).Discussion
Our results showed that the severity of WMH was an independent risk factor for MCI patients. We also found that MCI group showed significantly increased MD and RD, and significantly decreased MK, AK, RK, FA and KFA values in left hippocampus region. In left hippocampus region, FA, MK, RK, and KFA were significant positively correlated with MoCA score, while MD and RD were significant negatively correlated with MoCA score. Similar to our results, early studies comparing MCI, AD, and controls also found decreased values of MK in MCI and AD [10,11]. The decrease of MK, AK, RK, FA and KFA and elevated MD and RD suggested the gray matter microstructure may changes in the medial temporal cortex. This may be due to the loss of neuron cell bodies, synapses and dendrites, the extracellular spaces were increased, which further resulted in elevated mean diffusivity and radial diffusivity. The microstructural changes in left hippocampus were more obvious than in the right, this may due to the hippocampus cortex shows asymmetry in normal people, and the left hippocampus cortex may be more vulnerable.Conclusion
DKI technique can be used to probe the microstructural changes of hippocampus in MCI patients with cSVD. The DKI-derived parameters are feasible evaluation of patients with MCI in clinical situations.Acknowledgements
No acknowledgement found.References
1.Tabatabaei-Jafari, H., Shaw, M.E., and Cherbuin, N. Cerebral atrophy in mild cognitive impairment: A systematic review with meta-analysis. Alzheimers Dement (Amst) (2015)1(4), 487-504.
2.Tuladhar, A.M., van Norden, A.G., de Laat, K.F., Zwiers, M.P., van Dijk, E.J., Norris, D.G., et al. White matter integrity in small vessel disease is related to cognition. Neuroimage Clin (2015)7, 518-524.
3.Liu, D., Wang, Z., Shu, H., and Zhang, Z. Disrupted white matter integrity is associated with cognitive deficits in patients with amnestic mild cognitive impairment: An atlas-based study. SAGE Open Med (2016)4, 2050312116648812.
4. Gerstenecker, A., Hoagey, D.A., Marson, D.C., and Kennedy, K.M. White Matter Degradation is Associated with Reduced Financial Capacity in Mild Cognitive Impairment and Alzheimer's Disease. J Alzheimers Dis (2017)60(2), 537-547.
5. Hoy, A.R., Ly, M., Carlsson, C.M., Okonkwo, O.C., Zetterberg, H., Blennow, K., et al. Microstructural white matter alterations in preclinical Alzheimer's disease detected using free water elimination diffusion tensor imaging. PLoS One (2017)12(3), e0173982.
6. Wang, S., Yuan, J., Guo, X., Teng, L., Jiang, H., Gu, H., et al. Correlation between prefrontal-striatal pathway impairment and cognitive impairment in patients with leukoaraiosis. Medicine (Baltimore) (2017)96(17), e6703.
7. Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., et al. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage (2002)15(1), 273-289.
8. Lu, J., Li, D., Li, F., Zhou, A., Wang, F., Zuo, X., et al. Montreal Cognitive Assessment in Detecting Cognitive Impairment in Chinese Elderly Individuals: A Population-Based Study. Journal of Geriatric Psychiatry and Neurology (2012)24(4), 184-190.
9. Ashburner, J. SPM: a history. Neuroimage (2012)62(2), 791-800.1.
10.Gong, N.J., Wong, C.S., Chan, C.C., Leung, L.M., and Chu, Y.C. Correlations between microstructural alterations and severity of cognitive deficiency in Alzheimer's disease and mild cognitive impairment: a diffusional kurtosis imaging study. Magn Reson Imaging (2013)31(5), 688-694.
11.Falangola, M.F., Jensen, J.H., Tabesh, A., Hu, C., Deardorff, R.L., Babb, J.S., et al. Non-Gaussian diffusion MRI assessment of brain microstructure in mild cognitive impairment and Alzheimer's disease. Magn Reson Imaging (2013)31(6), 840-846.
Figures