1552
Strain-specific disease progression patterns of sporadic Creutzfeldt-Jakob disease revealed by Subtype and Stage Inference model1Neuroradiology Unit, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy, 2Centre for Medical Image Computing, Department of Computer Science, University College London, London, United Kingdom, 3Department of Neuroimaging, Institute of Psychiatry, Psychology and Neuroscience, King′s College London, London, United Kingdom, 4National Prion Disease Pathology Surveillance Center, Case Western Reserve University, School of Medicine, Cleveland, OH, United States, 5Department of Pathology, Case Western Reserve University, School of Medicine, Cleveland, OH, United States
Synopsis
Transmission studies in animal models have identified four strains of sporadic Creutzfeldt-Jakob disease (sCJD). Using a data-driven approach, we aim to identify subgroups of sCJD patients with distinct diffusion-weighted MRI (DWI) abnormality patterns, and test their association with disease strains. We used an unsupervised machine-learning algorithm named Subtype and Stage Inference, that identified 5 clusters of patients each having a distinct pattern of DWI abnormality progression: one had initial involvement of the parieto-frontal cortex; two started with subcortical regions (striatum, thalamus and cerebellum); and two had cortical and limbic regions affected early. Data-driven subgroups were significantly associated with sCJD strains.
INTRODUCTION
Human prion diseases comprise several subtypes, with different disease durations, clinical and histopathological features.1 Sporadic Creutzfeldt-Jakob disease (sCJD) is the most common form of prion disease, and its phenotypical heterogeneity is mostly determined by the type (1 or 2) of the pathogenic prion protein (PrPD) that causes the disease, coupled with the genotype (MM, MV or VV) at codon 129 of the prion protein gene.2 The experimental transmission of human prion disease to hosts has led to the identification of different sCJD strains named M1 (MM1 and MV1 subtypes), V2 (MV2K and VV2), M2C (MM2C and MV2C), and V1 (VV1 alone).3Among different MRI modalities, diffusion-weighted MRI (DWI) is the most sensitive to specific brain microstructural alterations caused by PrPD, such as spongiform degeneration, that appear with increased signal hyperintensities in the images.4 Our hypothesis is that there could be similarities between the phenotypic heterogeneity of sCJD observed at MRI and at neuropathology. However, the DWI hyperintensity pattern may change with disease progression, introducing a temporal heterogeneity between patients as they could be at different stages of the disease.5
In order to disentangle the phenotypic and temporal heterogeneity of sCJD, we aim to identify subgroups of patients with similar progression patterns of DWI abnormalities, using a novel machine-learning algorithm that learns joint spatiotemporal clusters of individuals from cross-sectional data. We hypothesise that the comparison between data-driven subgroups and sCJD subtypes will show for the first time the presence of strain-specific DWI progression patterns.
METHODS
MRIs of 412 autopsy-confirmed sCJD and 120 non-prion disease patients (control group) were examined by one neuroradiologist, scoring the DWI hyperintensities of 12 brain regions on an integer scale from “zero” (no CJD-related abnormality) to “three” (extensive CJD-related abnormality with decreased diffusivity on apparent diffusion coefficient maps). Selected brain regions were frontal, temporal, occipital, parietal, precuneus, cingulate, insula, hippocampus, caudate, putamen, thalamus, and cerebellum.To separate temporal and phenotypic heterogeneity, we adopted Subtype and Stage Inference (SuStaIn) model, an unsupervised machine-learning technique that uncovers population subgroups with common patterns of biomarker evolution.6 SuStaIn determines the optimal number of subgroups (clusters) based on the available imaging data (subtype/strain not included in the model) and simultaneously reconstructs the sequence of biomarkers becoming abnormal that defines each subgroup.
The clustering problem is solved by splitting clusters recursively, starting with a single cluster, until a maximum number of clusters is reached. The optimal number of clusters is estimated via model selection criteria obtained from cross-validation. The optimization of the sequences within each cluster is obtained by expectation-maximization involving the data likelihood, assuming a discrete uniform probability for the scores of each brain region.
RESULTS
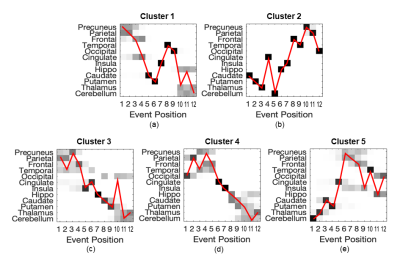
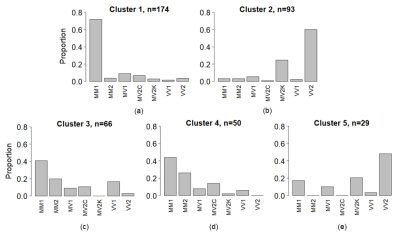
SuStaIn identified 5 clusters of sCJD patients with distinct sequences of progression (Figure 1a-e, from 1st to 5th cluster, respectively).The 1st cluster had an initial involvement of parietal and frontal cortices, followed by cingulate, striatum, and eventually thalamus and cerebellum. It was composed predominantly of MM1 subtype (Figure 2a).
The 2nd was the opposite of the 1st group, starting from the striatum, thalamus and cerebellum, and reaching the neocortex only at the last stages. VV2 and MV2K subtypes were highly represented in the 2nd cluster (Figure 2b).
The 3rd and 4th clusters had almost the same DWI progression pattern: neocortical regions were first involved, followed by limbic structures, while striatum, thalamus and cerebellum were the last. The difference between these two sequences is that the occipital cortex was the last region (in the 3rd cluster) and the first (in the 4th cluster) to become abnormal. These two clusters had similar distributions of MM/MV1, MM/MV2C and VV1 subtypes, with almost none MV2K or VV2 (Figure 2c,d).
Finally, the 5th group resembled the ordering of the 2nd group, but with a first involvement of the cerebellum, and an earlier involvement of the parietal region. VV2 and MV2K were the most represented subtypes in this cluster (Figure 2e).
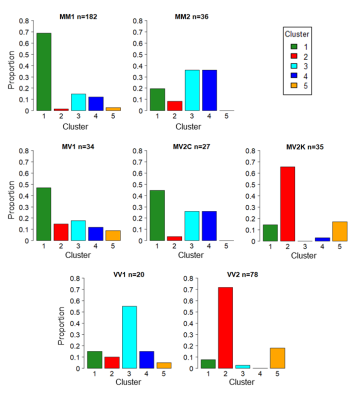
A significant association was found in the distribution of sCJD subtypes to the 5 clusters (p<0.0001, Pearson’s chi-squared test): most of the MM1 and MV1 patients were assigned to cluster 1; the majority of MM/MV2C were equally assigned to clusters 3 and 4; VV1 had a preferential association with cluster 3, and MV2K and VV2 were assigned to cluster 2 and, less frequently, to cluster 5 (Figure 3).
DISCUSSION & CONCLUSION
In this study we have demonstrated the presence of at least three specific DWI progression patterns in sCJD: (i) a parieto-frontal exordium with early involvement of the striatum in one group (cluster 1) associated to MM/MV1 subtype (M1 strain); (ii) an initial subcortical involvement in two groups (clusters 2 and 5), with striatum or cerebellum being the first regions to become abnormal, associated to MV2K and VV2 (V2 strain); (iii) all cortical and limbic regions affected early in two groups (clusters 3 and 4), mostly associated to MM/MV2C (M2C strain) and in particular to VV1 subtype (V1 strain) when the occipital is the only cortical region to become affected at the last stages.In conclusion, we found that these DWI progression patterns are strongly linked to the strains of sCJD, suggesting that strain-specific features of the disease could be captured in vivo by MRI.
Acknowledgements
RP, NPO, DCA and AB are supported by the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 666992. ALY is supported by an MRC skills development fellowship. PG is supported by NIH R01 NS083687 and P01 AI106705, and Charles S. Britton Fund. JB and BSA are supported by CDC NU38CK000480.References
- Puoti G, Bizzi A, Forloni G, Safar JG, Tagliavini F, Gambetti P. Sporadic human prion diseases: Molecular insights and diagnosis. Lancet Neurol. 2012;11(7):618-628.
- Gambetti P, Cali I, Notari S, Kong Q, Zou WQ, Surewicz WK. Molecular biology and pathology of prion strains in sporadic human prion diseases. Acta Neuropathol. 2011;121(1):79-90.
- Rossi M, Baiardi S, Parchi P. Understanding Prion Strains: Evidence from Studies of the Disease Forms Affecting Humans. Viruses. 2019;11(4):309.
- Manners DN, Parchi P, Tonon C, et al. Pathologic correlates of diffusion MRI changes in Creutzfeldt-Jakob disease. Neurology. 2009;72:1425-1431.
- Eisenmenger L, Porter MC, Carswell CJ, et al. Evolution of Diffusion-Weighted Magnetic Resonance Imaging Signal Abnormality in Sporadic Creutzfeldt-Jakob Disease, With Histopathological Correlation. JAMA Neurol. 2016;73(1):76-84.
- Young AL, Marinescu RV, Oxtoby NP, et al. Uncovering the heterogeneity and temporal complexity of neurodegenerative diseases with Subtype and Stage Inference. Nat Commun. 2018;9(1):4273.
Figures