Yang Wang1, Alexander Cohen1, Guanyu Chen2, Veena Nair3, Piero Antuono4, Malgorzata Franczak4, Vivek Prabhakaran3, Barbara Bendlin5, Shi-Jiang Li2, and the Alzheimer’s Disease Connectome Project6
1Radiology, Medical College of Wisconsin, Milwaukee, WI, United States, 2Biophysics, Medical College of Wisconsin, Milwaukee, WI, United States, 3Radiology, University of Wisconsin School of Medicine and Public Health, Madison, WI, United States, 4Neurology, Medical College of Wisconsin, Milwaukee, WI, United States, 5Medicine, University of Wisconsin School of Medicine and Public Health, Madison, WI, United States, 6Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
Measured using an advanced
3D pCASL with Hadamard encoded multiple PLDs, patients with MCI showed different patterns of
reduced CBF and prolonged ATT in comparison with both AD patients and healthy controls,
where CBF and ATT changes highly correlated with severity of disease as
assessed by neuropsychological test scores. These findings raised the
speculation of underlying vascular abnormality in prodromal AD. Our results
also suggested that ATT could serve as useful hemodynamic measure of itself,
may be of diagnostic utility for prodromal AD or vascular dementia.
INTRODUCTION
Alzheimer’s disease (AD) is characterized
by the accumulation of hyperphosphorylated tau and neurotoxic Aβ in the brain,
where hypoxia could stimulate this build-up of AD-specific proteins 1. While arterial spin labeling (ASL) perfusion
MRI is increasingly proving to be a promising tool for exploring neurovascular
changes in AD, the conventional 3D pCASL (pseudo-continuous ASL) with a single
post-labeling delay (PLD) is influenced by arterial transit time (ATT), i.e.
the time it takes for the blood to travel from the labeling slice to the brain
voxel of interest. Taking ATT into account can therefore reduce potential bias
when comparing cerebral blood flow (CBF) between groups. Furthermore, ATT is
interesting in itself, as it may reflect underlying pathologies2. Emerging evidence has shown reduced CBF in MCI and AD compared
to normal elderly adults, whereas ATT prolongation was also
observed in AD, although the ATT finding is less consistent across studies3. The neurovascular pathways to AD prognosis remain largely
unclear. This study was thus aimed to evaluate both CBF and ATT alterations in mild
cognitive impairment (MCI) and AD using an advanced 3D pCASL with Hadamard
encoded multiple PLDs.METHODS
90 participants were selected from the ADCP (Alzheimer’s Disease Connectome
Project) at both MCW and UW Madison sites, including 15 MCI patients, 11 AD
patients and 64 age-matched healthy controls (HC). All subjects underwent a MR
perfusion scan on 3T using a 3D pCASL sequence with Hadamard
encoded multiple PLDs (1.0s, 1.25s, 2.46s) with TR/TE as 9708/11.24ms and
voxel size of 1.875x1.875x4mm3. A long
(3.5s) labeling block was divided into three sub-boluses and four images were
acquired with control and label sub-boluses corresponding to the Hadamard
matrix. This was followed by a segmented stack of spirals readout. A raw
magnetization (M0) image was collected on the last repetition. Images for each
of the three delays were extracted using a linear combination of the four
images. After reconstruction, individual ATT was
computed using the signal-weighted delay method4. CBF for each PLD was estimated using the one-compartment
model5. The ATT corrected CBF was then estimated6. Additional high-resolution
T1-weighted MRPAGE was acquired using parameters: TR/TE=5.36/2.412, Flip
angle=8, voxel size=0.5x0.5x0.8. In post-processing, MPRAGE image was first
segmented into gray matter, white matter and cerebrospinal fluid using FSL. The
M0 image was linearly registered to the MPRAGE. The CBF and ATT maps were
registered to the MPRAGE using the M0 to MPRAGE transformation matrix. The
MPRAGE image was then registered to MNI space using ANTS and the M0, CBF, and ATT
images were registered to MNI (Montreal Neurological Institute) space using the
MPRAGE to MNI transformation matrix. Individual CBF and ATT maps were analyzed
for the group difference in the frame of general linear model. Age and sex were
used as covariates whenever appropriate to control for potential confounds.RESULTS
While
no group difference in age, gender and years of education was detected,
significant difference in the MoCA (Montreal Cognitive Assessment) score was found
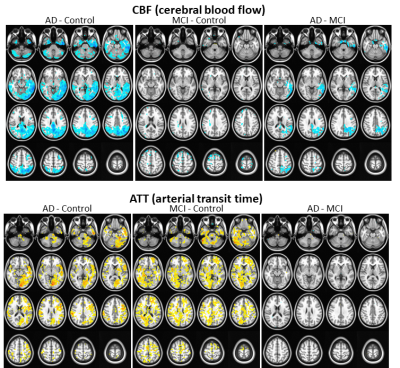
(HC > MCI > AD, p<0.0001). At the threshold of p<0.01 (FWE
corrected), significant reduction in CBF demonstrated in different areas in the
voxel-wise group comparisons of MCI vs. HC, AD vs. MCI, as well as AD vs. HC
(Fig 1). Furthermore, significantly increased ATT was detected in different
areas in group comparison of MCI vs. HC and AD vs. HC, but no difference in AD
vs. MCI (Fig. 1). Further
region-of-interest (ROI) analysis was conducted using a template of functional
networks7. Significant
correlations were detected between the MoCA score and ROI averaged CBF and ATT
in most regions, where the highest correlation was found in default mode
network (DMN) for both CBF (r=0.793, p<0.001) and ATT (r=-0.685,
p<0.005). Stepwise multiple regression analysis showed that 76.8% variance
of MoCA score in MCI and AD could be explained by DMN averaged CBF and ATT
combined, where decreased DMN CBF or increased DMN ATT significantly predicted the
lower MoCA score in MCI and AD patients.DISCUSSION
In accord with previous reports 1,3, we
found AD with reduced CBF and prolonged ATT compared to HC. Moreover, MCI
showed intermediate changes in reduced CBF between AD and HC groups, but very similar
patterns of increased ATT as that in AD, which indicated possible different
trajectory of ATT and CBF during the prognosis in prodromal AD. While less CBF was
observed secondary to aberration in metabolism commonly seen in MCI and AD,
diffuse ATT elevation in prodromal AD might lead to the speculation of
underlying vascular impairment. Recent highlights from the Alzheimer’s Disease
Neuroimaging Initiative suggested that data-driven AD progression models
supported multifactorial interactions rather than a linear cascade of events8, where vascular pathology
burden may act through both Aβ dependent and independent mechanisms to
exacerbate AD progression8. Further investigation is
warranted to validate our findings in longitudinal studies.CONCLUSION
Reduction in regional CBF are an essential feature of AD and have been
linked to cerebral amyloid-deposition. Using advanced 3D pCASL with multiple
PLDs to characterize neurovascular changes including both CBF and ATT has
important clinical implications to help classify patients with respect to their
disease signature so that specific pathologies, including vascular pathways,
can be therapeutically targeted1.Acknowledgements
This study is part of the Alzheimer’s Disease Connectome Project supported by National Institute on Aging (UF1AG051216).References
1. Strickland S. Blood will out:
vascular contributions to Alzheimer's disease. The Journal of clinical investigation. 2018;128(2):556-563.
2. Alsop DC, Detre JA, Golay X, Gunther
M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, van
Osch MJ, Wang DJ, Wong EC, Zaharchuk G. Recommended implementation of arterial
spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM
perfusion study group and the European consortium for ASL in dementia. Magnetic resonance in medicine : official
journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic
Resonance in Medicine. 2015;73(1):102-116.
3. Mak HK, Chan Q, Zhang Z, Petersen
ET, Qiu D, Zhang L, Yau KK, Chu LW, Golay X. Quantitative assessment of
cerebral hemodynamic parameters by QUASAR arterial spin labeling in Alzheimer's
disease and cognitively normal Elderly adults at 3-tesla. J Alzheimers Dis. 2012;31(1):33-44.
4. Dai W, Robson PM, Shankaranarayanan
A, Alsop DC. Reduced resolution transit delay prescan for quantitative
continuous arterial spin labeling perfusion imaging. Magnetic Resonance in Medicine. 2012;67(5):1252-1265.
5. Dai W, Garcia D, de Bazelaire C,
Alsop DC. Continuous flow-driven inversion for arterial spin labeling using
pulsed radio frequency and gradient fields. Magnetic
Resonance in Medicine. 2008;60(6):1488-1497.
6. Van der Thiel M, Rodriguez C,
Giannakopoulos P, Burke M, Lebel RM, Gninenko N, Van De Ville D, Haller S.
Brain perfusion measurements using multidelay arterial spin-labeling are
systematically biased by the number of delays. American Journal of Neuroradiology. 2018;39(8):1432-1438.
7. Yeo BT, Krienen FM, Sepulcre J,
Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L,
Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human
cerebral cortex estimated by intrinsic functional connectivity. Journal of neurophysiology. 2011;106(3):1125-1165.
8. Veitch
DP, Weiner MW, Aisen PS, Beckett LA, Cairns NJ, Green RC, Harvey D, Jack CR,
Jagust W, Morris JC, Petersen RC, Saykin AJ, Shaw LM, Toga AW, Trojanowski JQ.
Understanding disease progression and improving Alzheimer's disease clinical
trials: Recent highlights from the Alzheimer's Disease Neuroimaging Initiative.
Alzheimer's & Dementia. 2018.