1548
Grey matter volume alterations in trigeminal neuralgia: A systematic review and meta-analysis of voxel-based morphometry studies1Department of Radiology, The Affiliated Hospital of Southwest Medical University, Luzhou, China, 2Department of Anesthesiology, The Affiliated Hospital of Southwest Medical University, Luzhou, China
Synopsis
In recent decades, a growing number of structural neuroimaging studies of grey matter (GM) in trigeminal neuralgia (TN) have reported inconsistent alterations. We carried out a systematic review and meta-analysis to identify consistent and replicable GM volume abnormalities in TN patients. Our findings provide a thorough profile of GM volume alterations in TN patients and constitute robust evidence that aberrant GM volumes in the brain regions regulating and moderating sensory-motor and affective processing may play an important role in the pathophysiology of TN.
Synopsis
In recent decades, a growing number of structural neuroimaging studies of grey matter (GM) in trigeminal neuralgia (TN) have reported inconsistent alterations. We carried out a systematic review and meta-analysis to identify consistent and replicable GM volume abnormalities in TN patients. Our findings provide a thorough profile of GM volume alterations in TN patients and constitute robust evidence that aberrant GM volumes in the brain regions regulating and moderating sensory-motor and affective processing may play an important role in the pathophysiology of TN.Purpose
To identify consistent and replicable GM volume abnormalities and explore the potential moderators between clinical characteristics and GM volume alterations in TN patients.Methods
A systematic search was conducted for the relevant studies with voxel-wise analysis of the GM volume alterations in TN patients. Effect-size signed differential mapping (ES-SDM) was applied to analyse the GM volume differences between TN patients and healthy controls. Meta-regression analysis was used to explore the effects of clinical characteristics on GM volume alterations in TN patients.Results
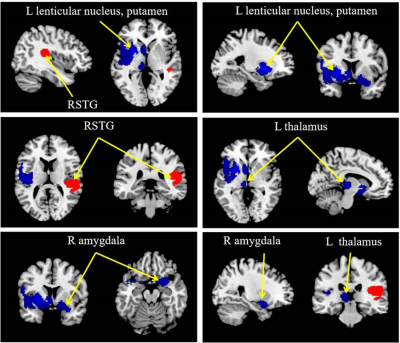
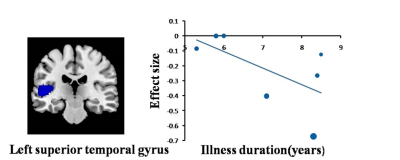
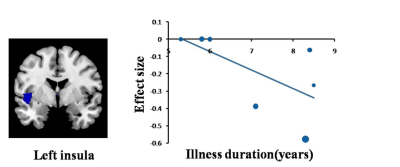
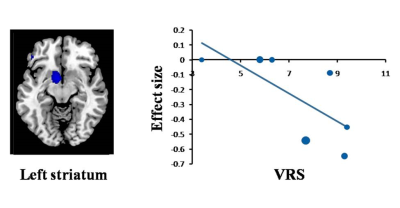
A total of 13 studies with 15 datasets, representing 407 TN patients and 376 healthy individuals, were included in the present study. The results revealed that TN patients had GM volume abnormalities mainly in the basal ganglia, including the putamen, nucleus accumbens (NAc), caudate nucleus and amygdala, as well as the cingulate cortex (CC), thalamus, insula and superior temporal gyrus (STG) , as shown in Fig. 1. Whole-brain jack-knife sensitivity analysis demonstrated that the loss of GM volume in the left lenticular nucleus in TN patients was highly repeatable, which was present in all datasets. The right superior longitudinal fasciculus remained significant in all but one combination of the datasets. The results in the right amygdala and left thalamus remained significant in all but two combinations. The meta-regression analysis showed that GM volume reductions in the left STG and insula were negatively correlated with the illness duration of the TN patients, as shown in Figs. 2 and 3. GM volume reductions in the left striatum were negatively correlated with the VRS scores, as shown in Fig. 4.Discussion
Emerging evidence has demonstrated that alterations in brain structure may be related to the pathophysiology of TN. However, the cerebral changes associated with TN are not yet fully understood1. Previous structural neuroimaging studies of GM in TN have reported inconsistent alterations2-6. Therefore, a systematic review and meta-analysis is emergently needed to identify the most prominent and consistent GM volume abnormalities and generate new insights into the pathophysiology of TN. Our findings revealed GM volume abnormalities located in the basal ganglia, CC, thalamus, insula and STG in patients with TN. Additionally, our study found that GM volume abnormalities in the left striatum, STG and insula may be potential neurobiological markers of the symptom ratings and illness duration of the disease.Conclusion
These results provide a thorough profile of GM volume alterations in TN patients and robust evidence that aberrant GM volumes in the brain regions regulating and moderating sensory-motor processing and affective processing may play an important role in the pathophysiology of TN.Acknowledgements
No acknowledgementsReferences
1. Love, S., Coakham, H.B., 2001. Trigeminal neuralgia: pathology and pathogenesis. Brain 124, 2347-2360.
2. Gustin, S.M., Peck, C.C., Wilcox, S.L., Nash, P.G., Murray, G.M., Henderson, L.A., 2011. Different pain, different brain: thalamic anatomy in neuropathic and non-neuropathic chronic pain syndromes. J Neurosci 31, 5956-5964.
3. Henderson, L.A., Peck, C.C., Petersen, E.T., Rae, C.D., Youssef, A.M., Reeves, J.M., Wilcox, S.L., Akhter, R., Murray, G.M., Gustin, S.M., 2013. Chronic pain: lost inhibition? J Neurosci 33, 7574-7582.
4. Obermann, M., Rodriguez-Raecke, R., Naegel, S., Holle, D., Mueller, D., Yoon, M.S., Theysohn, N., Blex, S., Diener, H.C., Katsarava, Z., 2013. Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. Neuroimage 74, 352-358.
5. Zhong, J., Chen, D.Q., Hung, P.S., Hayes, D.J., Liang, K.E., Davis, K.D., Hodaie, M., 2018. Multivariate pattern classification of brain white matter connectivity predicts classic trigeminal neuralgia. Pain 159, 2076-2087.
6. Tsai, Y.H., Yuan, R., Patel, D., Chandrasekaran, S., Weng, H.H., Yang, J.T., Lin, C.P., Biswal, B.B., 2018. Altered structure and functional connection in patients with classical trigeminal neuralgia. Hum Brain Mapp 39, 609-621.
Figures