1546
Exploration of mannitol-treated dehydration in acute stroke using diffusion kurtosis imaging with free water elimination1Institute of Biomedical Engineering and Nanomedicine, National Health Research Institutes, Miaoli, Taiwan, 2Institute of Neuroscience and Medicine – 4, Medical Imaging Physics, Forschungszentrum Jülich, Jülich, Germany, 3Department of Computer Science, National Chiao Tung University, Hsinchu, Taiwan, 4Center for Neuropsychiatric Research, National Health Research Institutes, Miaoli, Taiwan, 5Department of Medical Imaging, Taipei City Hospital, Taipei, Taiwan, 6Department of Computer Science and Information Engineering, Chang Gung University, Taoyuan, Taiwan, 7Department of Medical Imaging, National Taiwan University Hospital, Taipei, Taiwan, 8Institute of Medical Device and Imaging, National Taiwan University College of Medicine, Taipei, Taiwan
Synopsis
Brain swelling typically occurs in acute stroke.1 Mannitol, as a hyperosmolar agent, enables to effectively treat the increased intraocular pressure and cerebral edema in brain injury.2,3 Diffusion kurtosis imaging with free water elimination (DKI-FWE)4 has been recently reported the ability to assess diffusion indices by separating free water compartment in simulations and healthy volunteers. The purpose of the study was to examine the effect of mannitol infusion using a stroke rat model at acute and chronic stages assessed by DKI-FWE and DKI. Our preliminary results revealed that mean kurtosis (MK) was sensitive to reflect mannitol-treated dehydration in acute stroke rat.
Introduction
Brain swelling typically occurs in acute stroke.1 The administration of hyperosmolar agents, such as mannitol, allows to effectively reduce intraocular pressure and cerebral edema by extracting both intracellular and interstitial water in brain injury.2,3 Recently, Diffusion Kurtosis Imaging with free water elimination (DKI-FWE),4 extending DKI,5 has been reported in simulation and healthy volunteers, showing the capability of separating the signal contributions of free water and tissue for accurate estimates of diffusion characteristics and free water fraction. Although potentially useful, the capability of DKI-FWE on neurological diseases has not been investigated yet. Therefore, this study aims to employ DKI-FWE and DKI techniques to explore the mannitol-treated dehydration within brain lesions in acute and chronic stages of stroke. We attempted to examine if the mean kurtosis (MK) index could reveal the dehydration in lesions and compare the sensitivities of conventional and FWE models of DKI technique.Methods
Three adult male Sprague–Dawley rats, weighting 350-450 g, were underwent the right middle cerebral artery occlusion for 90 minutes as described.6 Rat was treated with a single dose of 20 % mannitol solution7 through intravenous injection. The first MRI scan was performed before mannitol injection and under the same experimental setup, the second scan starts at 20 minutes after mannitol injection. Experiments were performed on an in-house 3T MRI system.8 An ultra-high-strength gradient coil with a maximum strength of 675 mT/m (Resonance Research Inc., MA, USA) and a custom transmit/receive surface coil were utilized. Diffusion-weighted images (DWI) and T2-weighted images (T2WI) were acquired. For DWI, a Stejskal-Tanner EPI pulse sequence was implemented with the b-value (s/mm2)/number of diffusion directions: 500/12, 1000/26, and 2000/40. Eight b=0 images were obtained. The sequence parameters were TR/TE of 2000/50 ms, Δ/δ of 24/3 ms, 1 average, slice thickness of 1 mm, FOV of 25 × 25 mm2, matrix size of 96 × 96, and 20 slices. T2WI was acquired using fast spin-echo sequence with in-plane resolution of 130 × 130 μm2 for examination of brain infarction. All datasets were first for denoising.9 Diffusion tensors and diffusion kurtosis tensors were reconstructed with DKI5 and DKI-FWE4 model analyses in Matlab. For DKI-FWE, the diffusivity of free water was fixed at 3 μm2/ms. Non-linear least-squares fitting was used. Regions-of-interest (ROIs) of lesion were determined on hyper-intensity regions in T2WI. ROI analysis was performed on MK and mean diffusivity (MD) maps from both conventional and FWE models. The percentage change of indices were quantified to evaluate the sensitivities to detect the dehydration in lesions.Results
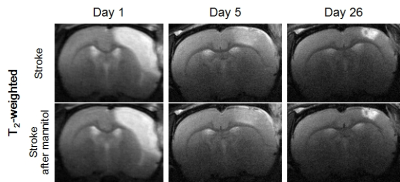
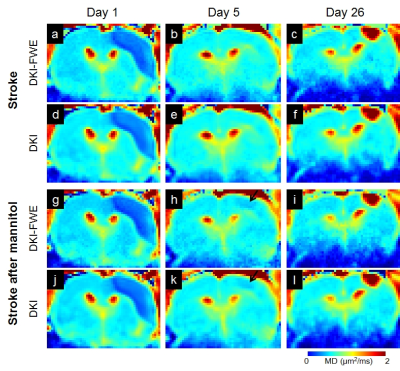
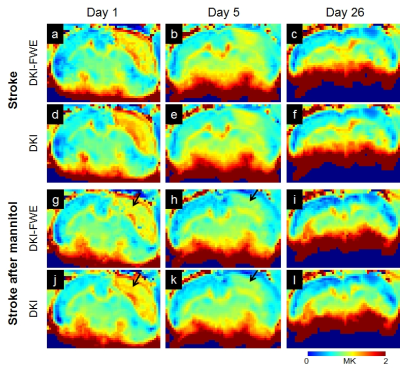
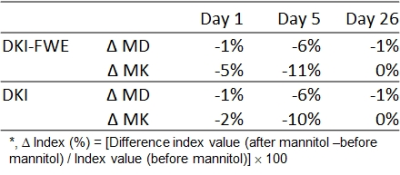
Figure 1 shows cerebral infarction of stroke rats in T2WI. For each time point, there was no obvious decrease of lesion size in stroke after mannitol infusions. Figure 2 shows, with time course, both DKI and DKI-FWE analyses indicated MD of lesions was decreased on day 1, close to normal on day 5, and elevated on day 26. MD was slightly decreased by using DKI-FWE model, compared to conventional DKI model. With mannitol administration, our results indicated that MD was apparently decreased on day 5 (~6%) from both analyses (h and k, arrows), compared to that on day 1 and 26 (~1%). Figure 3 shows both analyses indicated that MK of lesions was apparently increased particularly on day 1 and day 5. MK then became much smaller at chronic stage. DKI-FWE, compared to DKI, indicated an incremental decrease of MK on day 1 (~3% for stroke, a vs. d; ~6% for stroke after mannitol, g vs. j), but revealed similar or slightly increased MK on day 5 (b vs. e and h vs. k) and day 26 (c vs. f and i vs. l). In stroke lesions, DKI-FWE revealed different patterns of MK on day 1 (a vs. d), but not MD (a vs. d in Figure 2). After mannitol administration, MK was obviously decreased on day 1 (~5% from DKI-FWE and ~2% from DKI; g and j, arrows) and on day 5 (~11% from DKI-FWE and ~10% from DKI; h and k, arrows), but almost unchanged on day 26. Table 1 lists the percentage changes of MD and MK before and after mannitol injection.Discussion and Conclusion
DKI-FWE, compared to conventional DKI, revealed decreases of MD and MK in stroke lesions, reflecting the removal of water contamination, consistent with Collier et al., 2018. With mannitol administration, our results indicated that MK was apparently decreased on day 1 and day 5 from DKI-FWE model. However, the conventional DKI analysis only showed the decrease on day 5. This may suggest that DKI-FWE has the higher sensitivity to reflect dehydration in stroke lesions. Interestingly, at chronic stage, the percentage changes of MD and MK show a very minor change before and after mannitol injection. One possible explanation is that, due to blood-brain barrier (BBB) integrity at chronic stage, mannitol may remain in vascular compartment and show a slower dehydration, compared to acute stage with damaged BBB. In summary, our preliminary results show MK could reflect the dehydration in stroke lesion and the DKI-FWE model could provide higher sensitivity than conventional DKI model. For verifying our current observations, the number of rats has to be increased and a more sophisticated analysis is needed in further studies.Acknowledgements
This work was supported by the National Health Research Institutes (108-PP-06) and Ministry of Science and Technology (108-2221-E-400-002 and 108-2911-I-400-502) in Taiwan.References
1. Bansal S et al., Drug Treatment of Acute Ischemic Stroke. Am J Cardiovasc Drugs. 2013; 13(1): 10.1007/s40256-013-0007-6.
2. Knapp JM, Hyperosmolar therapy in the treatment of severe head injury in children: mannitol and hypertonic saline. AACN Clin Issues. 2005; 16(2):199-211.
3. Diringer MN et al., Osmotic therapy. Neurocritical Care. 2004; 1(2):219-233.
4. Collier Q et al., Diffusion Kurtosis Imaging With Free Water Elimination: A Bayesian Estimation Approach. Magnetic Resonance in Medicine. 2018; 80:802–813.
5. Jensen JH et al., MRI Quantification of Non-Gaussian Water Diffusion by Kurtosis Analysis. NMR Biomed. 2010; 23(7): 698–710.
6. Wu KJ et al., Transplantation of Human Placenta-Derived Multipotent Stem Cells Reduces Ischemic Brain Injury in Adult Rats. Cell Transplantation. 2015; 24(3): 459–470.
7. Paczynski RP et al., Multiple-Dose Mannitol Reduces Brain Water Content in a Rat Model of Cortical Infarction. Stroke. 1997; 28(7):1437–1444.
8. Cho KH et al., Development, integration and use of an ultrahigh-strength gradient system on a human size 3T magnet for small animal MRI. PLoS ONE. 2019; 14(6):e0217916.
9. Manjon JV et al., Adaptive Non-Local Means Denoising of MR Images with Spatially Varying Noise Levels. Journal of Magnetic Resonance Imaging. 2010; 31(1):192–203.
Figures