1544
Investigation of amide proton transfer imaging in Multiple system atrophy at 3.0 Tesla1Department of Radiology, Beijing Hospital, National Center of Gerontology, Beijing, China, 2Graduate School of Peking Union Medical College, Beijing, China, 3Department of Neurology, Beijing Hospital, National Center of Gerontology, Beijing, China, 4GE Healthcare, MR Research China, Beijing, China
Synopsis
Multiple system atrophy (MSA) is in great need of diagnosis in its early stage. Our study aims to evaluate the feasibility of using amide proton transfer (APT) imaging in detection of multiple system atrophy (MSA) at 3.0 Tesla. We found that APT MTRasym values were significantly higher in MSA patients than in normal controls at red nucleus, substantia nigra, thalamus and putamen. We also found that APT MTRasym values were significantly higher in probable MSA than in possible MSA patients at red nucleus, caudate and putamen. Hence, CEST may be valuable in diagnosing and predicting the progression of MSA.
Synopsis
Multiple system atrophy (MSA) is in great need of diagnosis in its early stage. Our study aims to evaluate the feasibility of using amide proton transfer (APT) imaging in detection of multiple system atrophy (MSA) at 3.0 Tesla. We found that APT MTRasym values were significantly higher in MSA patients than in normal controls at red nucleus, substantia nigra, thalamus and putamen. We also found that APT MTRasym values were significantly higher in probable MSA than in possible MSA patients at red nucleus, caudate and putamen. Hence, CEST may be valuable in diagnosing and predicting the progression of MSA.Introduction
Multiple system atrophy (MSA) is an adult-onset, health-threatening, sporadic, progressive neurodegenerative disorder[1]. Therefore, there is a great clinical need for early diagnosis of MSA. Previous studies have shown that amide proton transfer (APT) imaging at 3.0T has the potential clinical value in diagnosis of brain tumors[2]and Parkinson’s disease[3]. Due to the fact that abnormal cytoplasmic protein accumulation, gliosis and microgliosis have been found in MSA patients[1] , we hypothesis that these pathological changes may lead to modified APT effects. Using the APT chemical exchange saturation transfer (CEST) imaging, here we aim to evaluate the feasibility of APT in diagnosis of MSA.Methods
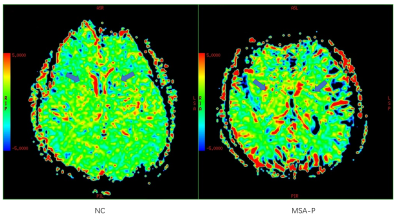
Twenty MSA-parkinsonian type (MSA-P) patients (10 male and 10 female; age range: 60-81 years) and twenty age-matched normal controls (8 male and 12 female; age range: 55-82 years) were enrolled in this study. In MSA-P patients, fourteen patients were diagnosed as clinically probable MSA-P patients, and six patients were diagnosed as clinically possible MSA-P. All data were obtained on a 3.0T MR scanner (SIGNA Pioneer, GE Healthcare). All participants underwent T2-weighted imaging (T2WI) and APT CEST acquisition with following parameters: two transverse slices of the head including the midbrain and the basal ganglia, TR/TE = 2500/26.2 ms, flip angle = 20°, FoV = 240×240 mm, matrix = 128×128, slice thickness = 5 mm, NEX = 2. The frequency offsets acquired were: 5000 1996 768 640 576 512 480 448 416 384 320 256 192 128 96 64 32 0 -32 -64 -96 -128 -192 -256 -320 -384 -416 -448 -480 -512 -576 -640 -768 Hz. Magnetization transfer ratio asymmetry (MTRasym) was further calculated on the AW4.6 GE Workstation. Six regions of interest (ROIs) including red nucleus, substantia nigra, globus pallidus, thalamus, caudate and putamen were manually drawn based on T2WI images. Mann-Whitney U test was used to compare the MTRasym (3.5ppm) difference between all MSA-P patients and normal controls, and between probable and possible MSA-P patients.Results
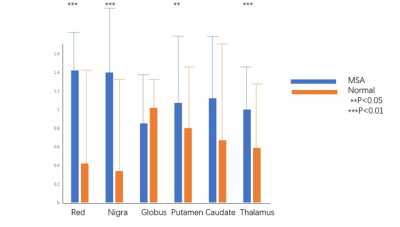
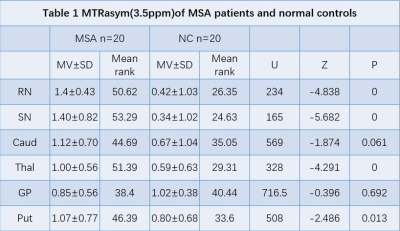
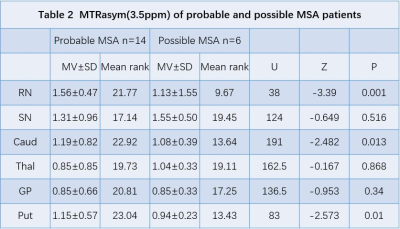
Figure 1 shows the comparison of MTRasym values between all MSA-P patients and normal controls in six ROIs. Specifically, we found that MSA-P patients have significantly higher MTRasym values at red nucleus (p = 0.000), substantia nigra (p = 0.000), thalamus (p = 0.000), and putamen (p = 0.013)(Table 1,Figure 2). Table 2 shows the comparison of MTRasym values between probable and possible MSA-P patients. Significantly higher MTRasym values at red nucleus (p = 0.001), caudate (p = 0.013), and putamen (p = 0.010) were observed in probable MSA-P patients than in possible MSA-P patients.Discussion and Conclusion
The preliminary results show that the APT MTRasym of the red nucleus, substantia nigra, thalamus and putamen were higher in MSA-P patients than in normal controls. The observations may be attributed to the pathophysiological mechanism of MSA-P. In MSA-P, the primary pathological impairment regions are striatonigral pathway, which may lead to pathological abnormalities of the substantia nigra, red nucleus, thalamus, and putamen[4].Our findings of a higher MTRasym in MSA-P patients suggest that the accumulation of abnormal cytoplasmic protein, gliosis and microgliosis were severer than neuron loss on account of most of the MSA-P patients in our group.For comparison of APT MTRasym between probable MSA and possible MSA-P patients, no significant difference was observed in substantia nigra, which indicated the pathological manifestation of the substantia nigra may be the same in both MSA-P sub-groups. The higher APT MTRasym values at red nucleus, caudate, and putamen in probable MSA patients may be associated with more cytosolic proteins and peptides deposition at these regions. Overall, our findings suggest that APT CEST may be valuable in diagnosing and predicting the progression of MSA.Acknowledgements
No acknowledgement found.References
[1]. Nagai, Y., et al., Multiple-system atrophy in long-term professional painter: a case report. Case Reports in Pathology,2012,(2012-05-28), 2012. 2012(1): p. 613180.
[2]. Khlebnikov, V., et al., Amide proton transfer (APT) imaging of brain tumors at 7 T: The role of tissuewater T1 -Relaxation properties. Magnetic resonance in medicine, 2017. 77(4): p. 1525-1532.
[3]. Li, C., et al., Chemical Exchange Saturation Transfer MRI Signal Loss of the Substantia Nigra as an Imaging Biomarker to Evaluate the Diagnosis and Severity of Parkinson'sDisease. Frontiers in neuroscience, 2017. 11: p. 489.
[4]. Tetsutaro, O., et al., The spectrum of pathological involvement of the striatonigral and olivopontocerebellar systems in multiple system atrophy: clinicopathological correlations. Brain A Journal of Neurology, 2004. 127(12): p. 2657-71.
Figures