1543
Nucleus Accumbens Deep Brain Stimulation Ameliorated Cognitive Impairment in Metabolic Syndrome Animal Model
Ting-Chieh Chen1, Yu-Chun Lo2, Szu-Yi Chou2, Ssu-Ju Li1, Ting-Chun Lin1, Ching-Wen Chang1, Yin-Chieh Liu1, Hsin-Tzu Lu1, and You-Yin Chen1,2
1Biomedical Engineering, National Yang-Ming University, Taipei, Taiwan, 2Ph.D. Program for Neural Regenerative Medicine, Taipei Medical University, Taipei, Taiwan
1Biomedical Engineering, National Yang-Ming University, Taipei, Taiwan, 2Ph.D. Program for Neural Regenerative Medicine, Taipei Medical University, Taipei, Taiwan
Synopsis
Cognitive dysfunctions were demonstrated to be associated with the metabolic syndrome (MetS), which could be treated with deep brain stimulation (DBS) of nucleus accumbens (NAc) by altering brain circuitss and facilitating synapse plasticity. However, NAc-DBS for memory-related cognitive function has yet to be investigated. Diffusion MRI, resting-state functional MRI, and behavioral test were applied in this study. We found restoration of the microstructure, increased functional connectivity, and an improvement in cognitive behaviors after NAc-DBS in the MetS models, C-C motif ligand 5/Regulated-on-Activation-Normal-T-cell-Expressed-and-Secreted knockout mice.
Introduction
Cognitive dysfunctions were associated with the metabolic syndrome (MetS)1. MetS and altered mitochondria dynamics were correlated with the neurodegenerative progresses2. The dopaminergic pathways were sensitive to glucose metabolism in the whole brain3, which was mainly composed of the mesolimbic and the mesocortical pathways4. The mesolimbic pathway transmitted dopamine from the ventral tegmental area (VTA) to the limbic system via the nucleus accumbens (NAc), integrating the signals from limbic and cortical regions5. Besides, the mesocortical pathway transmitted dopamine from the VTA to the downstream regions, the prefrontal cortex (PFC)5. NAc was responsible for cognitive functions, including reward functions, executive functions, and long-term memory6-8 , and played a crucial role in the dopaminergic system. Deep brain stimulation (DBS) targeting NAc demonstrated increased dopamine efflux with antidepressant-like behavior by altering mitochondrial function in the energy metabolism9. However, NAc-DBS for memory-related cognitive function has yet to be investigated. In this study, we applied NAc-DBS treatment, resting-state functional MRI (rsfMRI), diffusion tensor imaging (DTI), and behavioral test to the C-C motif ligand 5 (CCL5)/Regulated-on-Activation-Normal-T-cell-Expressed-and-Secreted (RANTES) knockout (KO) mice, the MetS model10. We hypothesized that DBS of the NAc may restore the functional connectivity (FC) and the white matter (WM) integrity at dopaminergic pathways, which thereby improves cognitive functions.Methods
Male adult C57BL/6 mice, which were used as healthy controls, and male adult CCL5/RANTES KO mice (Jackson Laboratory Inc.; MGI:2180195; Bar Harbor, ME, USA) (weight 25 ± 5 g), which were used as MetS models in the study, were housed in the animal facility under 12:12-h light/dark cycle with controlled temperature at 22 ± 2°C. To investigate the NAc-DBS therapeutic effect on the MetS mice, the animals were divided into three groups: (1) healthy controls (N = 5) (2) CCL5/RANTES KO sham controls (N = 5) (3) CCL5/RANTES KO DBS group (N = 5). All mice were implanted with MR-compatible neural probes into the bilateral NAc (AP: + 1.1 mm, ML: ± 1.1 mm, DV: − 4.0 mm) in week 10. The bilateral NAc-DBS (frequency:130 Hz; pulse width 60 μs; intensity 200 μA) was applied consecutively for 30 minutes to CCL5/RANTES KO DBS group per day in week 13 (Figure 1). Novel object recognition (NOR) test was conducted to evaluate long-term recognition memory in week 12 (pre-DBS) and week 13 (post-DBS), respectively11, and a preference index (PI) was calculated as the memory performance. Whole brain images were acquired from a 7 Tesla Bruker MRI scanner (Bruker Biospec 70/30 USR, Ettlingen, Germany) in week 14. The rsfMRIs were acquired using gradient-echo planar imaging (GE-EPI) sequence (TR / TE = 2,000 / 20 ms, FOV = 20 × 20 mm2, matrix size = 80 × 80, bandwidth = 200 kHz, 14 coronal slices, and thickness = 0.5 mm). DTIs were acquired by the DTI EPI Spin-Echo sequence (TR / TE = 3750 / 0.4 ms, FOV = 20 × 20 mm2, matrix size = 50 × 50). Regions of interest (ROIs) were selected according to the Allen mouse brain atlas12, including medial prefrontal cortex (mPFC), NAc, hippocampus (HIPP), and VTA (Figure 2A). FC was calculated through the FMRIB Software Library v5.0 (FSL 5.0; http://www.fmrib.ox.ac.uk/fsl) and the analysis of functional neuroimages (AFNI) software (http://afni.nimh.nih.gov/afni), then normalized to the baseline (healthy controls). DTI analysis was performed by DSI Studio (http://dsi-studio.labsolver.org), and the fractional anisotropy (FA) values were averaged within each ROI. For the visualization of FC, significant connections were identified by using a one sample t test (FDR-corrected p < 0.05 thresholds with the minimum cluster size of 200 voxels). The Kruskal-Wallis test (p < 0.05) was conducted to compare FC values, FA values and the PI of NOR among the three groups. A post-hoc analysis with Dunn’s test (p < 0.05) was performed to elucidate the within-group difference.Results
A week after the CCL5/RANTES KO mice received NAc-DBS, increased FC was observed in the VTA and in downstream region among mPFC, NAc and HIPP (Figure 2B & 2C). FA values in the HIPP and VTA also exhibited significant increase in the CCL5/RANTES KO DBS group compared to the sham controls (Figure 3). Moreover, PI in CCL5/RANTES KO DBS group was increased to the baseline, suggesting that NAc-DBS could ameliorate the cognitive impairment in CCL5/RANTES KO mice (Figure 4).Discussion
FC between the dopaminergic pathways was enhanced after NAc-DBS in the CCL5/RANTES KO mice. Dopamine neurons played a fundamental role in motivational and memory processes for the ability to modulate synaptic transmission and plasticity13, 14. Additionally, decreased FA values were assumed to result from degradation in myelinated fibers14. Therefore, cognitive abnormality was restored to the baseline after NAc-DBS in the CCL5/RANTES KO mice due to increased FC and FA values in dopaminergic regions and HIPP.Conclusion
The findings implied that NAc-DBS could alter the WM in the dopaminergic pathways and strengthen the memory-related cognitive behavior, and suggested that the NAc-DBS may be a potential therapeutic intervention for memory disabilities in MetS models.Acknowledgements
No acknowledgement found.References
- Dik, M.G., et al., Contribution of metabolic syndrome components to cognition in older individuals. Diabetes care, 2007. 30(10): p. 2655-2660.
- Jha, S.K., et al., Linking mitochondrial dysfunction, metabolic syndrome and stress signaling in Neurodegeneration. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 2017. 1863(5): p. 1132-1146.
- de Araujo, I.E., X. Ren, and J.G. Ferreira, Metabolic sensing in brain dopamine systems, in Sensory and Metabolic Control of Energy Balance. 2011, Springer. p. 69-86.
- Alcaro, A., R. Huber, and J. Panksepp, Behavioral functions of the mesolimbic dopaminergic system: an affective neuroethological perspective. Brain research reviews, 2007. 56(2): p. 283-321.
- Floresco, S.B., C.L. Todd, and A.A. Grace, Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. Journal of Neuroscience, 2001. 21(13): p. 4915-4922.
- Ikemoto, S., Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory. Neuroscience & biobehavioral reviews, 2010. 35(2): p. 129-150.
- Kerfoot, E.C. and C.L. Williams, Contributions of the Nucleus Accumbens Shell in Mediating the Enhancement in Memory Following Noradrenergic Activation of Either the Amygdala or Hippocampus. Frontiers in Pharmacology, 2018. 9: p. 47.
- Yamagata, N., et al., Distinct dopamine neurons mediate reward signals for short-and long-term memories. Proceedings of the National Academy of Sciences, 2015. 112(2): p. 578-583.
- Kim, Y., et al., Nucleus accumbens deep-brain stimulation efficacy in ACTH-pretreated rats: alterations in mitochondrial function relate to antidepressant-like effects. Translational psychiatry, 2016. 6(6): p. e842.
- Chou, S.-Y., et al., CCL5/RANTES contributes to hypothalamic insulin signaling for systemic insulin responsiveness through CCR5. Scientific reports, 2016. 6: p. 37659.
- Leger, M., et al., Object recognition test in mice. Nature protocols, 2013. 8(12): p. 2531.
- Lein, E.S., et al., Genome-wide atlas of gene expression in the adult mouse brain. Nature, 2007. 445(7124): p. 168.
- Thierry, A.M., et al., Hippocampo‐prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus, 2000. 10(4): p. 411-419.
- Snow, W.M., et al., In vivo detection of gray matter neuropathology in the 3xTg mouse model of Alzheimer’s disease with diffusion tensor imaging. Journal of Alzheimer's Disease, 2017. 58(3): p. 841-853.
Figures
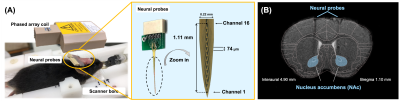
Figure1. (A) Experimental
setup of animal MRI measurement. The neural probes were implanted into the
bilateral NAc then bent into 90 degrees, fixing with dental cement. The overall
view of the neural probe assembly with 16 channels and spacing of 74 µm. (B)
The mice brain atlas was overlaid on T2-weighted image to show the track of the
neural probes implanted into the bilateral NAc (marked in the blue area).
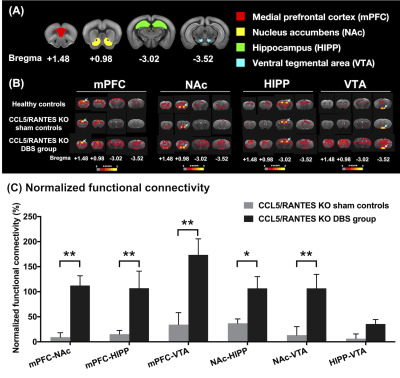
Figure2.
(A)
Four ROIs in this study including mPFC (red), NAc (yellow), HIPP (green), and
VTA (blue). (B) The maps that corresponded to rsfMRI for different ROIs
demonstrated on mPFC, NAc, HIPP and VTA (highlighted with blue arrows in the
brain atlas). (C) The CCL5/RANTES KO mice after NAc-DBS showed significant
restoration of FC compared with the CCL5/RANTES KO sham controls. *:
p < 0.05; **: p < 0.01
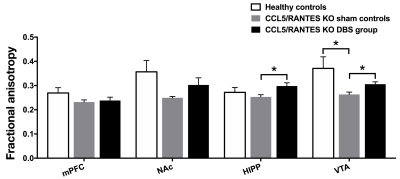
Figure3.
The
CCL5/RANTES KO mice after NAc-DBS showed significant restoration of FA in HIPP
and VTA compared with the CCL5/RANTES KO sham controls. *: p < 0.05
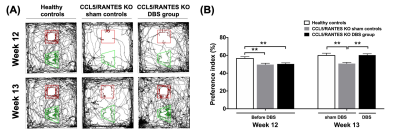
Figure4.
(A) The
tracking visualization results showed the CCL5/RANTES KO sham controls spent
more time exploring familiar object (red square) than novel object (green
v-shape). On the other hand, the CCL5/RANTES KO mice after NAc-DBS spent more
time exploring novel object like healthy controls. (B) At the week 13 after
NAc-DBS treatment, the PI in the CCL5/RANTES KO DBS group become higher and
reach to the baseline (healthy controls). **: p < 0.01