1540
Cerebral Blood Flow and Cerebrovascular Reactivity in Acute and Chronic HIV-Infection Treated by Combination Antiretroviral Therapies1Physics and Astronomy, University of Rochester, Webster, NY, United States, 2Neurology, University of Rochester, Rochester, NY, United States, 3Imaging Sciences, University of Rochester, Rochester, NY, United States, 4Electrical and Computer Engineering, University of Rochester, Rochester, NY, United States, 5Biostatistics and Computational Biology, University of Rochester, Rochester, NY, United States, 6Microbiology and Immunology, University of Rochester, Rochester, NY, United States, 7Microbiology, Immunology and Tropical Medicine, The George Washington University, Washington DC, DC, United States
Synopsis
Combination antiretroviral therapy (cART) maintains virologic control in HIV patients, but may lead to neurotoxicity. By using neuroimaging and cellular microparticle quantification, we explore the effects cART may have in both acute and chronic HIV-infection. We find that cART treatment does reduce microparticle levels associated with neuroinflammation to those of controls. Further, microparticle levels and neuroimaging results strengthen assumptions about immune dysfunction in HIV infection. We demonstrate that cerebral blood flow and cerebrovascular reactivity can be used in conjunction with quantitative microparticle levels to study the effects of neuroinflammation and cART treatment in both acute and chronic HIV infection.
Introduction
The introduction of combination antiretroviral therapy (cART) has dramatically increased the life expectancy of individuals with HIV-infection. Despite regulating inflammation in the presence of HIV, cART may lead to central nervous system (CNS) toxicity, albeit studies suggesting this lacked objective neuroimaging data and an appropriate control population1,2. One study implies the possible effect on the cerebrovascular function given the negative impact of cART on carotid arteries due to aging3. Cerebral blood flow (CBF) and cerebrovascular reactivity (CVR) as measured by arterial spin labeling (ASL) and functional MRI (fMRI) are linked to vascular pathology in HIV-infection via neural activation and vascular resistance4. Here we use neuroimaging, astrocytic microparticles (MP) containing Sonic Hedgehog (Shh+) molecules, and endothelial MP markers to preliminarily assess vascular changes associated with CNS toxicity in both acute and chronic HIV-infection.Methods
In this ongoing study about neurotoxicity in an HIV population on cART, 88 subjects (mean± SD age = 37.8 ± 12.6 years, range = 18 – 63 years) were evaluated to study the effects of neurotoxicity of cART (Table 1). All imaging was conducted on a 3T (Siemens TIM TRIO) scanner equipped with a 32-channel head coil (Erlangen, Germany). The protocol includes high-resolution T1-weighted anatomical images using the MPRAGE sequence (TI=1,100ms, TE/TR=3.44ms/2,530ms, 1mm isotropic resolution). ASL data was collected using a pseudo-continuous ASL sequence (TE/TR=22.62ms/3,530ms, PLD=1.5s, 36 repetitions, 3.8x3.8x5mm3 resolution). fMRI was collected with an echo-planar imaging (EPI) sequence (TE/TR=30ms/2,000ms, 150 volumes, 4mm isotropic resolution). CBF was calculated using oxford_asl5. Relative CVR indices were calculated at each voxel using the global signal regressor method6. Images were registered to the T1w and MNI152 spaces using FMRIB’s Software Library’s FLIRT and FNIRT tools7. The Harvard-Oxford atlases were used to calculate region averages in the caudate (CAU), putamen (PUT), globus pallidus (PAL), thalamus (THA), and precuneus (PUC). MPs were measured by volumetric flow cytometry analysis using Accuri C6 in a subset of HIV patients and age-matched controls before and after 12 weeks of cART treatment. Statistical analyses included two sample t-tests to detect CBF and CVR mean differences between HIV cohorts and time points in each region, correlations between imaging and MP markers, the Kruskal-Wallis test followed by Dunn’s multiple comparison correction, and multivariate regressions to evaluate the effects of cART in acute and chronic HIV treatment, controlling for age, gender, and HIV status.Results
ROIs and example CBF and CVR maps are shown in figure 1.Neuroimaging
In the acute and chronic regression models, age was significantly associated with CBF decrease in every ROI (p<=0.0003) and gender was significant in the caudate (p=0.0004) and thalamus (p=0.0004). The chronic model also showed that HIV-status was positively related to CBF (p<=0.009), but the interaction of HIV and time was negatively related to CBF (p<=0.046) in every ROI. Long-term effects models showed that age is associated with increased CVR (p<=0.03) but decreased CBF (p<=0.01) in every ROI.
Microparticles
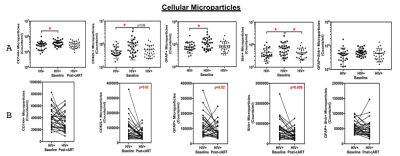
MPs of astrocyte origin (glial fibrillary acidic protein, GFAP+), endothelial origin (CD144+) and those derived from leukocytes (CD62L+) showed significantly increased levels in HIV infected individuals prior to initiation of cART as compared to controls. Post-cART, these changes were normalized to the levels detected in controls (Figure 2). Further, correlations between MPs carrying singular markers Shh+, CD62L+, or GFAP+, and CVR revealed significant positive relationships in the PUC, CAU, PAL, and PUT in controls. Similarly, Shh+ or CD62L+ MPs were positively correlated with CBF in the PUC and CAU in controls. None of the MP markers were correlated with imaging markers in the HIV-infected cohort except dual GFAP+Shh+ with CVR in the PUT.
Discussion
AcuteOur results demonstrate that cART treatment can reduce the levels of the MPs to levels equivalent to controls. Imaging markers show that age and gender are important covariates, rather than HIV-infection. We expect that while subjects remain on cART, MP levels will mimic controls. Imaging and MP correlations suggest that CVR decreases with immune dysfunction. Further blood analyses should confirm this.
Chronic
In the chronic regression, we found that HIV-infection increases CBF for up to two years compared to controls, despite virologic maintenance. We also found a significant negative interaction of HIV-infection and time, potentially indicating that chronic HIV-infection or cART treatment impacts CBF. Overall, CVR is unaffected by gender and HIV status. Without removing possible biasing effects of vascular risk factors, these results are consistent with a healthy population despite the presence of HIV. These early results imply that cART may have an effect on vascular health as shown by CBF decrease longitudinally that should be confirmed with more rigorous testing.
Conclusion
In this preliminary analysis, we have shown that MPs reflect the acute nature of HIV-infection and HIV has a chronic impact on CBF measures, thus implying cART affects neural function despite maintaining virologic control. Further blood samples and more comprehensive statistical analyses are required to strengthen these early results.Acknowledgements
This work was made possible by the NIH 5R01MH099921-05. We would also like to acknowledge the study coordinators and study participants.
References
1. Schweinsburg B, Taylor M, Alhassoon O, Gonzalez R, Brown G, Ellis R, Letendre S, Videen J, McCutchan J, Patterson T, and Grant I. Brain Mitochondrial Injury in Human Immunodeficiency Virus-Seropositive (HIV+) Individuals Taking Nucleoside Reverse Transcriptase Inhibitors. J.Neurovirol.11(4):356-64.16. 2005.
2. Tardieu M, Brunelle F, Raybaud C, Ball W, Barret B, Pautard B, Lachassine E, Mayaux M, and Blanche S. Cerebral MR Imaging in Uninfected Children Born to HIV-Seropositive Mothers and Perinatally Exposed to Zidovudine. AJNR: American Journal of Neuroradiology26(4):695-701. 2005.
3. Singh M, Kotla S, Le N, Ae Ko K, Heo K, Wang Y, Fujii Y, Thi Vu H, McBeath E, Thomas T, Jin Gi Y, Tao Y, Medina J, Taunton J, Carson N, Dogra V, Doyley M, Tyrell A, Lu W, Qiu X, Stirpe N, Gates K, Hurley C, Fujiwara K, Maggirwar S, Schifitto G, Abe J. Senescent Phenotype Induced by p90RSK-NRF2 Signaling Sensitizes Monocytes and Macrophages to Oxidative Stress in HIV-Positive Individuals. Circulation. 139(9):1199-216. Epub 2018/12/28. 2019.
4. Ances B, Sisti D, Vaida F, Liang C, Leontiev O, Perthen J, Buxton R, Benson D, Smith D, Little S, Richman D, Moore D, and Ellis R. Resting Cerebral Blood Flow: a Potential Biomarker of the Effects of HIV in the Brain. Neurology73(9):702-8. 2009.
5. Chappell M, Groves A, Whitcher B, Woolrich M. Variational Bayesian inference for a non-linear forward model. IEEE Transactions on Signal Processing 57(1):223-236, 2009.
6. Liu P, Li Y, Pinho M, Park D, Welch B, & Lu H. Cerebrovascular reactivity mapping without gas challenges. NeuroImage, 146, 320–326. 2017.
7. Woolrich M, Jbabdi S, Patenaude P, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith S. Bayesian analysis of neuroimaging data in FSL. NeuroImage, 45:S173-86, 2009.
Figures

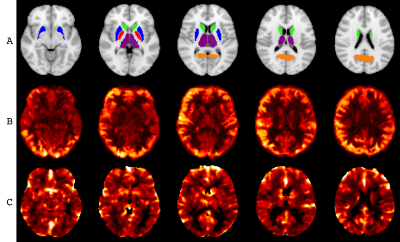
Figure 1. A. ROIs considered: THA – purple, PUC – orange, PUT – blue, PAL – red, CAU – green. B. Example CBF map for one control subject, range 0 – 100 mL/100g tissue. C. Example CVR map for one control subject, range 0 – 2 %BOLD change.