1531
Patterns of regional cortical thinning in cognitively impaired patients with Parkinson’s disease1Department of NMR & MRI Facility, All India Institute of Medical Sciences, New Delhi, India, 2Department of Neurology, All India Institute of Medical Sciences, New Delhi, India, 3National Drug Dependence Treatment Centre, All India Institute of Medical Sciences, New Delhi, India, 4Department of Biostatistics, All India Institute of Medical Sciences, New Delhi, India, 5Department of Psychiatry, All India Institute of Medical Sciences, New Delhi, India, 6Department of Physiology, All India Institute of Medical Sciences, New Delhi, India
Synopsis
Cognitive impairment (CI) affects 20-40% Parkinson’s disease (PD) patients. Cortical thickness (CT), measuring the shortest distance between brain surface and inner edge of cortical gray matter may relate to CI in PD. In this study, we evaluated CT alteration in cognitively impaired PD patients (PD-CI) in comparison to cognitively normal (CN) healthy controls (HC) and PD patients (PD-CN) using 3DT1 MR data. Extended cortical thinning in frontal, temporal, parietal, occipital regions in PD-CI and significant positive association with global cognition MoCA score may signify cognition linked Lewy pathology and may be a promising tool to characterize cognition in PD.
Introduction
Parkinson’s disease (PD), clinically marked by motor dysfunction, presents diverse spectrum of non-motor symptoms including cognitive impairment, autonomic dysfunction, psychiatric disturbances and sensory disorders. 20-40% of PD patients get affected by cognitive deficits with overall 83% of patients develop dementia within 20 years of diagnosis [1,2]. Lewy body cortical involvement in PD was postulated to initiate in the temporal mesocortex (stage 4), reaching the prefrontal cortex (stage 5) and finally involving the primary, sensory and premotor areas [3]. Though, involvement of Lewy bodies in neuronal degeneration is not established, pathological cortical changes may relate to cognitive impairment in PD. Cortical thickness (CT), measuring shortest distance between brain surface and inner edge of cortical gray matter, may directly project the cortical alterations and hence, may be a promising tool to investigate morphological brain changes [2]. Hence, in the present study, we tested the hypothesis of cortical thickness alterations in cognitively impaired patients with PD (PD-CI) in a comparative study with cognitively normal patients with PD (PD-CN) and cognitively normal healthy subjects (HC).Methods
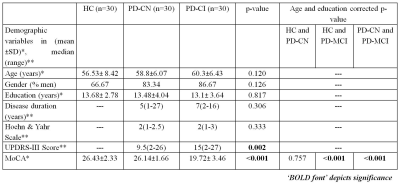
We recruited 30 HC (56.53±8.42 years), 30 PD-CN (58.8±6.07 years) and 30 PD-CI (60.3±6.43 years) subjects for the study. Cognitive categorization of subjects followed MoCA global cognitive screening test. MR scans were performed using a 32 channel head coil on a 3T MR scanner (Achieva 3.0T TX, Philips Medical Systems, The Netherlands) using three dimensional T1 weighted image (3D T1WI) with TR/TE=3/7ms, flip angle=8°, FOV=256x256mm, slice thickness=1mm, slices=180, and method=Turbo Field Echo. Statistical Parametric Mapping (version SPM 12) Computational Anatomy Toolbox (CAT 12) based on MatLab (R2018b) was used for cortical thickness based surface analysis using default pipeline on subject’s T1 data [4]. One way ANOVA with ‘age’ correction was utilized to estimate intergroup differences in CT. Multiple linear regression with education (duration) and age as covariates were used to establish correlation between CT and MoCA score in PD. ROC analysis using STATA (ver 12.1, SatCorp LP, USA) on extracted mean CT was performed to evaluate diagnostic ability of CT.Results
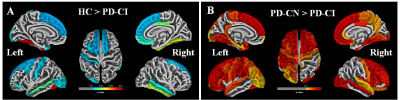
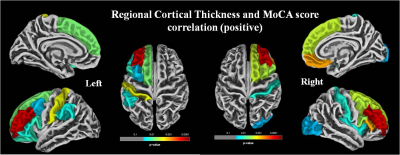
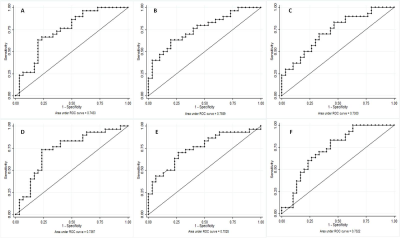
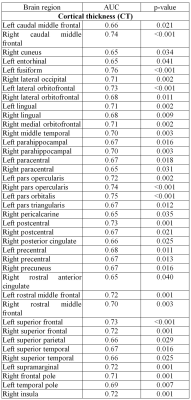
The global cognitive functioning was significantly affected in PD-CI with reduced MoCA score and widespread temporal, parietal, occipital and frontal cortical thinning in comparison to HC and PD-CN with p<0.05, Holm-Bonferroni corrected and p<0.05, FDR corrected respectively for whole brain (figure 1). No significant difference was observed between HC and PD-CN groups. CT and MoCA score was significantly correlated in the PD group (figure 2). ROC analysis showed multiple brain regions with significant AUC for CT in differentiating PD-CN and PD-CI (figures 3, 4).Discussion
In PD-CI, highly significant cortical thinning in temporal fusiform, middle, inferior temporal, temporal parahippocampal, orbitofrontal, frontal pars orbitalis, pars opercularis, parietal postcentral, cingulate, supramarginal regions signify alterations in multiple cognitive domains which was reflected in cognitive performance also (figure 1). UPDRS-III (severity of motor symptoms) score was higher in PD-CI compared to PD-CN implying shared neuropathology in cognitive and motor symptoms in PD [5]. Though, Lewy body pathology doesn’t fully explain cognitive impairment in PD [6], current cortical thinning pattern was consistent with Braak’s staging of PD pathology [3]. However, frontal atrophy may associate to amyloid β deposition featuring Alzheimer (AD) like pathology [7]. Research on PD dementia prediction should combine both Lewy and AD pathologies [6]. Bilateral frontal superior, middle, orbitalis, pars opercularis, pars triangularis and parietal supramarginal, postcentral CT positively correlated with the global cognitive MoCA score. ROC analysis of mean CT revealed alterations in PD-CI (with respect to PD-CN) in medial orbitofrontal, caudal and rostral middle frontal, frontal pars opercularis, pars orbitalis, superior frontal, middle temporal, fusiform, parahippocampal, parietal postcentral, supramarginal, lateral occipital and insula areas, similar to a previous study [8] . The fronto-parietal networks serve as a hub of cognitive control and altered regional CT may predict PD dementia. The regions share a high degree of functional connectivity to distributed cognitive networks and are crucial for optimal cognitive functionality [9]. Cortical thinning in fronto-parietal-temporal networks (involved in memory, executive functions, attention and visuospatial functions), suggest negative effect on cognitive task performance in PD-CI. Thus, brain cortical surface mantle gets altered in cognitively impaired patients with PD and longitudinal studies following these patterns of cortical thickness alterations may unravel cognitive correlates characterizing progression to dementia in PD.Conclusion
Pronounced temporal, frontal, and parietal cortical thinning in cognitively impaired patients with PD corresponds to Lewy body pathology and may be a potential MR imaging tool differentiating cognitive impairment in PD. CT may be promising in evaluating cognition linked pathology in PD.Acknowledgements
SC acknowledges fellowship from All India Institute of Medical Sciences, New Delhi, India.References
[1] Hou J-G G & Lai E C. Non-motor Symptoms of Parkinson’s Disease. Int. J. Gerontol. 2007, 1: 53–64.
[2] Zhang L, Wang M, Sterling NW, Lee EY, Eslinger PJ, Wagner D, Du G, Lewis MM, Truong Y, Bowman FD, Huang X. Cortical Thinning and Cognitive Impairment in Parkinson's Disease without Dementia. IEEE/ACM Trans Comput Biol Bioinform. 2018, 15:570-580.
[3] Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003, 24:197-211.
[4] Farokhian F, Beheshti I, Sone D, Matsuda H. Comparing CAT12 and VBM8 for Detecting Brain Morphological Abnormalities in Temporal Lobe Epilepsy. Front Neurol. 2017, 8:428.
[5] Wang Y-X, Zhao J, Li D-K, Peng F, Wang Y, Yang K, et al. Associations between cognitive impairment and motor dysfunction in Parkinson’s disease. Brain Behav. 2017, 7:e00719–e00719.
[6] Compta Y, Parkkinen L, O’Sullivan SS, Vandrovcova J, Holton JL, Collins C, et al. Lewy- and Alzheimer-type pathologies in Parkinson’s disease dementia: which is more important? Brain. 2011,134:1493–505.
[7] Yau Y, Zeighami Y, Baker TE, Larcher K, Vainik U, Dadar M, et al. Network connectivity determines cortical thinning in early Parkinson’s disease progression. Nat Commun [Internet]. 2018, 9:12.
[8] Zarei M, Ibarretxe-Bilbao N, Compta Y, Hough M, Junque C, Bargallo N, et al. Cortical thinning is associated with disease stages and dementia in Parkinson's disease. J Neurol Neurosurg &amp; Psychiatry [Internet]. 2013, 84:875 LP – 882.
[9] Marek S, Dosenbach NUF. The frontoparietal network: function, electrophysiology, and importance of individual precision mapping. Dialogues Clin Neurosci. 2018, 20:133–40.
Figures