1530
Prospective Longitudinal Diffusion Tensor Tractography Evidence of Nigrostriatal Degeneration in Early Parkinson’s Disease
Arthur Yong1, Amanda Lee2, Septian Hartono2,3, Isabel Chew2, Samuel Ng3, Xinyi Choi3, Wilson Jia Wei Wong2, Linda Soo Lee Lim4, Eng King Tan1,3, Louis Tan1,3, and Ling Ling Chan1,2
1Duke-NUS Graduate Medical School, Singapore, Singapore, 2Singapore General Hospital, Singapore, Singapore, 3National Neuroscience Institute, Singapore, Singapore, 4National Heart Centre Singapore, Singapore, Singapore
1Duke-NUS Graduate Medical School, Singapore, Singapore, 2Singapore General Hospital, Singapore, Singapore, 3National Neuroscience Institute, Singapore, Singapore, 4National Heart Centre Singapore, Singapore, Singapore
Synopsis
Parkinson's disease (PD) is characterized by progressive dopaminergic neuronal loss in the substantia nigra (SN) and dopaminergic deafferentation in the striatal nuclei. Diffusion tensor tractography (DTT) has been used to document cross-sectional changes in the nigrostriatal pathway (NSP) in PD. Our prospective longitudinal study using DTT revealed significant interval NSP degeneration over a two-year period compared to controls, besides cross-sectional changes in the NSP congruent with current literature. Our results suggested that demyelination may be the dominant factor in NSP degeneration in PD. DTT may be a useful objective biomarker of disease progression in the early stages of PD.
Introduction
Parkinson's disease (PD) is marked by progressive dopaminergic neuronal loss in the substantia nigra (SN) and dopaminergic deafferentation in the striatal nuclei. Cross-sectional studies have demonstrated sensitivity of diffusion tensor tractography (DTT) to neurodegeneration in the nigrostriatal pathway (NSP) in PD.1-4 In this prospective longitudinal study, we investigated the utility of DTT in documenting progressive changes in the NSP in patients with de novo PD.Methods
Institutional ethics approval was appropriately obtained. Seventy-one subjects (Figure 1) comprising 55 newly-diagnosed PD patients (M/F 31/24; mean age 64.0±7.6) and 16 age-matched healthy controls (HC, M/F 10/6; mean age 63.7±6.6) underwent two MRI scans two years apart, each on the same scanner using identical protocols. All subjects had their Hoehn and Yahr (H&Y) score and Unified Parkinson’s Disease Rating Scale (UPDRS) motor score charted prior to each scan.5,6All subjects underwent brain MRI on a Siemens Skyra 3T (Siemens Healthcare, Erlangen, Germany) scanner with a 32-channel head coil. Diffusion imaging was acquired with SE-EPI sequence with the following parameters: TR/TE=10118/102ms, matrix=122×122, slices=55, slice thickness=2.5mm, FOV=220mm×220mm, acquisition time=11:09mins, with 30 diffusion-encoding directions each at b-values=1000 and 2000s/mm2. Three non-diffusion-weighted volumes (b=0) were also acquired. T1-weighted MPRAGE scan was also performed with TR/TE=1900/2.44ms, TI=900ms, matrix=256×256, number of slice=256, slice thickness=1mm, FOV=250mm×250mm. Trace-weighted images and T1-weighted scans were screened to exclude pathology in the nuclei.
A tractography pipeline based on MRTrix was developed.7 Eddy current distortion and noise corrections were performed. Fiber orientation distributions estimation was done with constrained spherical deconvolution algorithm8 while the five tissue type image was generated for downstream anatomically constrained tractography (ACT)9 (Figure 2). Seed regions-of-interest (ROIs) were manually drawn in the SN, whilst the striatal nuclei ROIs were automatically segmented using FSL-FIRST10 (Figure 3). NSP tractography from ipsilateral SN to striatal nuclei was performed with probabilistic tractography using ACT. Comparison of DTI metrics (FA-fractional anisotropy, MD/AD/RD-mean/axial/radial diffusivity) between baseline and follow-up scan in PD and HC was performed.
Results
The PD patients and HC were age-matched and gender distribution did not differ significantly (Figure 1). Interval between scans was longer in the control group.There was significant progression of clinical motor scores in the PD cohort between baseline and two-year scan, more obvious on UPDRS assessment (Figure 1). Expectedly, significant differences in both H&Y & UPDRS clinical scores between PD and controls existed at both first and second timepoints. Representative image of NSP tracts is shown in Figure 4.There is a significant reduction in FA of NSPs to left and right globus-pallidus (GP), thalamus, and left putamen in PD after two years, whilst only FA of NSPs connecting to the right caudate, right GP and left putamen were decreased in controls (Figure 5A).
MD of NSPs connecting to left and right caudate, GP, putamen, as well as left thalamus were increased in PD after two years, whilst only that connecting to the right caudate nucleus showed an increase (Figure 5B) in controls.
AD of NSPs connecting to left and right caudate, putamen, left thalamus, and left globus pallidus also increased in PD after two years, whilst no significant change occurred for all nuclei in controls (Figure 5C).
RD of NSPs connecting to left and right caudate nuclei, putamen, thalamus, and right globus pallidus also increased in PD after two years, whilst only that connecting to the right caudate increased in controls (Figure 5C).
Discussion
Our results showed prevalent NSP tract degeneration across the basal ganglia nuclei in PD patients, as indicated by lower FA and higher diffusivity after two years, in agreement with our current understanding of the biopathology11 in PD. In contrast, such observations were scanty in the healthy controls. These findings suggested that greater NSP degeneration occurred over the two-year period in PD patients. Our findings also corroborated those of previous cross-sectional study1 which found NSP diffusivity increased and FA reduced in PD patients compared to healthy controls. Therein, the average disease duration of the PD group was 5.1 ± 2.9 years, and significantly longer than the disease duration of our current PD group who presented with de novo disease. This suggested that tracking NSP degeneration through DTT may potentially be a useful marker for PD diagnosis in the early stages of disease.Diffusivity changes were found in both axial and radial directions. Changes in AD are associated with axonal injury12, whilst changes in RD are associated with demyelination.12-14 Overall, we observed more changes in RD compared to AD. This indicated that demyelination may be the dominant factor in NSP degeneration in PD. Given the association between changes in RD and demyelination, further study using myelin water imaging may be useful to confirm these as the primary factor behind NSP degeneration in PD.
Conclusion
DTT detected longitudinal NSP degeneration in patients presenting with de novo PD after 2 years, but not in controls. There was increased diffusivity and reduced fractional anisotropy across various NSP tracts in PD, congruent with our understanding of NSP degeneration, and suggesting potential utility of DTT as an objective biomarker to track PD progression in early disease stages.Acknowledgements
We would like to thank the National Medical Research Council, Singapore for the funding support.References
- Tan WQ, Yeoh CS, Rumpel H, et al. Deterministic tractography of the nigrostriatal-nigropallidal pathway in Parkinson’s disease. Scientific reports. 2015; (5):17283.
- Zhang Y, Wu IW, Buckley S, et al. Diffusion tensor imaging of the nigrostriatal fibers in Parkinson's disease. Mov Disord. 2015;30(9):1229-36.
- Theisen F, Leda R, Pozorski V, et al. Evaluation of striatonigral connectivity using probabilistic tractography in Parkinson's disease. Neuroimage Clin. 2017;9(16):557-563.
- Andica C, Kamagata K, Hatano T, et al. Neurite orientation dispersion and density imaging of the nigrostriatal pathway in Parkinson's disease: Retrograde degeneration observed by tract-profile analysis. Parkinsonism Relat Disord. 2018;51:55-60.
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology. 1967;17(5):427-427.
- Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Movement disorders: official journal of the Movement Disorder Society, 2008;23(15):2129-2170.
- Tournier JD, Calamante F, Connelly A. MRtrix: Diffusion tractography in crossing fiber regions. Int J Imaging Syst Technol 2012;22:53–66.
- Tournier JD, Calamante F, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super- resolved spherical deconvolution. Neuroimage 2007;35(4):1459-1472.
- Smith, RE, Tournier JD, Calamante F, et al. Anatomically- constrained tractography: improved diffusion MRI streamlines tractography through effective use of anatomical information. Neuroimage 2012;62(3):1924-1938.
- Patenaude B, Smith SM, Kennedy D, et al. A Bayesian Model of Shape and Appearance for Subcortical Brain. NeuroImage 2011;56(3):907-922.
- Kalia LV, Lang AE. Parkinson disease in 2015: evolving basic, pathological and clinical concepts in PD. Nature reviews Neurology, 2016;12(2):65.
- Budde MD, Kim JH, Liang HF, et al. Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn Reson Med 2007;57(4):688-95.
- Song SK, Sun SW, Ramsbottom MJ, et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 2002;17(3):1429-1436.
- Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 2005;26:132-140.
Figures
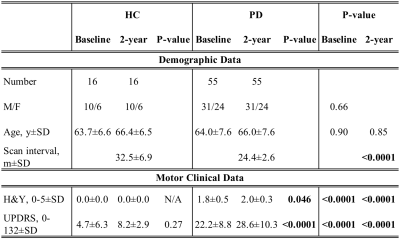
Figure 1. Study Demographics
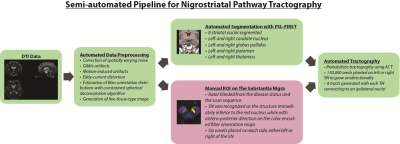
Figure 2. NSP tractography pipeline developed on MRTrix.
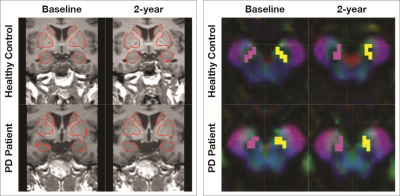
Figure 3. ROIs for probabilistic tractography of the nigrostriatal pathway. Automated segmentation of target ROIs in the basal ganglia (coronal view) overlaid on 3D structural MPRAGE sequence in (left) and manual seed ROI placement in the substantia nigra (axial view) on color FA map in (right).
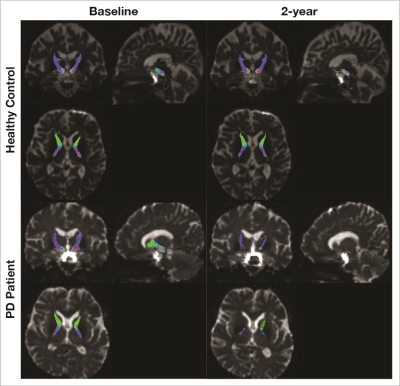
Figure 4. Sample images of NSP tracts in a healthy subject and PD patient at baseline and follow-up scan.
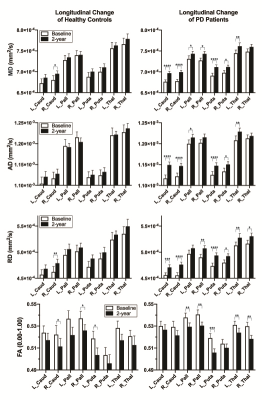
Figure 5. Comparison of DTT metrics in healthy controls and PD patients. *) p < 0.05, **) p < 0.01, ***) p < 0.001, ****) p < 0.0001.