1525
Free-water imaging in Substantia Nigra in Parkinson’s disease: A Neuromelanin Sensitive MRI Atlas Based Study
Apurva Shah1, Jacob Antony Alapatt2, Shweta Prasad3, Jitender Saini4, Pramod Pal3, Ragini Verma2, and Madhura Ingalhalikar1
1Symbiosis Center for Medical Image Analysis, Symbiosis International University, Pune, India, 2Department of Radiology, University of Pennsylvania, Philadelphia, PA, United States, 3Department of Neurology, National Institute of Mental Health and Neurosciences, Bengaluru, India, 4Department of Radiology, National Institute of Mental Health and Neurosciences, Bengaluru, India
1Symbiosis Center for Medical Image Analysis, Symbiosis International University, Pune, India, 2Department of Radiology, University of Pennsylvania, Philadelphia, PA, United States, 3Department of Neurology, National Institute of Mental Health and Neurosciences, Bengaluru, India, 4Department of Radiology, National Institute of Mental Health and Neurosciences, Bengaluru, India
Synopsis
Parkinson’s disease is characterized by degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNc). To study the micro-structural and free-water (FW) changes using diffusion-MRI in the SNc it is critical to extract SNc accurately. Our work employs a neuromelanin sensitive MRI based atlas to delineate the SNc and demonstrates significant FW and FW eliminated microstructural alterations in a large cohort of PD (with and without psychosis) and its association with PD severity, indicative of novel diagnostic and progression markers of PD which however demonstrate no role in genesis of psychosis in PD.
Introduction
Recent studies have demonstrated elevated levels of freewater (FW) in the substantia nigra (SN) in Parkinsons’s disease (PD) compared to healthy controls (HCs)1,2. The FW estimation is performed using a bi-tensor model that separates the derived diffusion MRI (dMRI) signal into isotropic (FW) and tissue-based components3. In application to PD, the FW is extracted from the small SN region embedded in the brain-stem, the localization of which is complicated and is generally performed manually4 or using T2 weighted or susceptibility weighted imaging (SWI). Manual delineation of SN suffers from low accuracy and reproducibility while using T2/SWI, as it primarily captures the SN pars reticulata (SNr), a result of iron sensitivity and not the SN pars compacta (SNc) which contains the dopaminergic neurons that are degenerated in PD5. Our work mitigates this problem by employing a neuromelanin sensitive MRI based atlas that can accurately delineate the SNc and computes FW and FW eliminated anisotropy and diffusivity measures using recent advancements in model initialization6 in a large cohort of PD. Moreover, we also investigate FW variation with disease severity and based on subtypes of patients with PD only and PD with psychosis (visual hallucinations).Method
Our study included 133 subjects with PD and 99 HC that were scanned on a Philips 3T Achieva MRI scanner. Diffusion-MRI images were acquire using 32 channel head coil with TR/TE = 8783/62 ms, field of view = 224 × 224 mm, voxel size = 1.75 × 1.75 × 2 mm, and no intersection gap. DTI was performed along 15 directions with a b value = 1000 s/mm2 and a single b = 0 s/mm2 image. Pre-processing included skull stripping, motion and eddy correction (using affine transformation). Free-water computation was performed using a bi-compartment model with isotropic (FW) compartment and the tissue compartment. The initialization was based on log-linear interpolation of mean diffusivity and mean signal from white matter and CSF from b=0 image facilitating a more stable fit6. The FW maps and the FW eliminated fractional anisotropy (FAt), axial diffusivity (AXt) and radial diffusivity (RDt) maps were computed. For SN localization, a recent atlas created from 27 controls using NMS-MRI, that accurately delineates the SNc was employed4. The regions were mapped to subject space using the Advanced Normalization Tool (ANTs) deformable registration7. To examine the group differences in FW levels, FAt, AXt and RDt, multivariate analysis of covariance (mancova) was performed between HC and PD as well as PD-psychosis and PD without psychosis with age and gender as covariates and correction for multiple comparisons was performed using false discovery rates (FDR) with a threshold of p<0.05. Similarly, test for laterality (R vs. L) was performed on the four measures in the PD group. To evaluate associations between the microstructural changes to the severity of the disease, the residuals from the FW and diffusion measures after regressing out age and gender were correlated to the Unified Parkinson’s Disease Rating Scale (UPDRS-III) OFF scores (where available) and duration of illness (DoI) of patients with PD.Results
Table 1 provides the demographic and clinical information. Figure 1 displays the SNc region of interest (probabilistic atlas) that captures the complete nigrosomes and the nigral matrix. The estimated mean FW in the SNc was significantly higher in both left (p<0.0001) and right (p<0.0001, corrected) in PD patients compared to controls with left FW significantly higher than right in PD patients (p<0.001, corrected). Interestingly, the FAt values were also significantly higher in PD patients than in controls (p<0.005 ,corrected) and the AXt demonstrated a similar trend (L-SNc: p=0.032, uncorrected, R-SNc: p=0.001, corrected) while RDt were higher in controls than in PD (L-SNc: p=0.026, uncorrected) (Figure 2). Figure 3 illustrates a slice with PD (high FW) and healthy control (with low FW) in the SNc. The correlation analysis illustrated a positive correlation between mean FAt and UPDRS-III OFF scores (p-value=0.019) as well as a trend between mean FW in right SNc and duration of illness (p-value=0.06) (Figure 4). Finally, no differences in all 4 measures were observed between the PD without psychosis group and PD-with psychosis.Discussion
Using accurate delineation of the SNc our work demonstrated that not only FW but a combination of the diffusion measures (FW, FAt, AXt and RDt) together may provide deeper insights into the PD pathology. Increase in FW may imply neuro-inflammation in the SNc while increase in FAt (with increased AXt and reduced RDt) may perhaps suggest axonal shrinkage or compression after eliminating all the FW and concurs with earlier FAt findings1,2. Significant asymmetry in FW was indicative of lateralized degeneration and was in accordance with clinical asymmetry that is typically observed in PD. Although FW increased with duration of illness and FAt with UPDRS, no differences between PD and PD-psychosis suggests that progression of PD could be independent of onset of psychosis symptoms although PD-psychosis is typically associated with later stages of illness. Overall, SNc diffusion measures could be considered a novel marker for PD and its progression however it may not play a significant role in the genesis of psychosis in PD.Acknowledgements
We would like to acknowledge Department of Science and Technology - Science Education and Research Board (DST-SERB) (EMR/2017/004523) for funding to this project. This work was also supported by the National Institutes of Health (NIH) [R01NS096606].References
- Ofori, E., Pasternak, O., Planetta, P. J., Burciu, R., Snyder, A., Febo, M., et al.. (2015). Increased free water in the substantia nigra of Parkinson's disease: a single-site and multi-site study. Neurobiology of aging, 36(2), 1097-1104.
- Ofori, E., Krismer, F., Burciu, R. G., Pasternak, O., McCracken, J. L., Lewis, M. M., et al.. (2017). Free water improves detection of changes in the substantia nigra in parkinsonism: A multisite study. Movement Disorders, 32(10), 1457-1464.
- Pasternak, O., Sochen, N., Gur, Y., Intrator, N., & Assaf, Y. (2009). Free water elimination and mapping from diffusion MRI. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 62(3), 717-730.
- Safai, A., Prasad, S., Saini, J., Pal, P. K., &Ingalhalikar, M. (2019). Microstructural Abnormalities of Substantia Nigra in Parkinson’s disease: A Neuromelanin Sensitive MRI Atlas Based Study. BioRxiv, 686154.
- Langley, J., Huddleston, D. E., Chen, X., Sedlacik, J., Zachariah, N., & Hu, X. (2015). A multicontrast approach for comprehensive imaging of substantia nigra. Neuroimage, 112, 7-13. doi:10.1016/j.neuroimage.2015.02.045
- Abdol Aziz Ould Ismail, Drew Parker, Moises Hernandez-Fernandez, Steven Brem, Simon Alexander, et al.. Characterizing Peritumoral Tissue Using Free Water Elimination in Clinical DTI. MICCAI 2018 - 21st International Conference on Medical Image Computing and Computer Assisted Intervention ; Workshop : Brain Lesion, Sep 2018, Granada, Spain. pp.1-9.
- Xiao, Y., Lau, J. C., Anderson, T., DeKraker, J., Collins, D. L., Peters, T., & Khan, A. R. (2019). Bridging micro and macro: accurate registration of the BigBrain dataset with the MNI PD25 and ICBM152 atlases. bioRxiv, 561118.
Figures
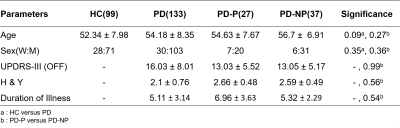
Table 1: Demographic and Clinical information
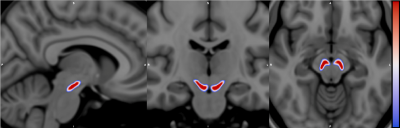
Figure 1 : NMS-MRI-27 probabilistic atlas for Substantia Nigra pars
compacta (SNc)
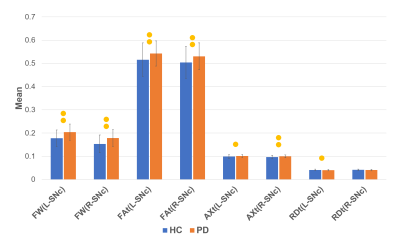
Figure
2: Chart demonstrating differences in HC and PD. The bars with two
dots indicate significance after FDR correction and bars with one dot
indicate uncorrected significance.
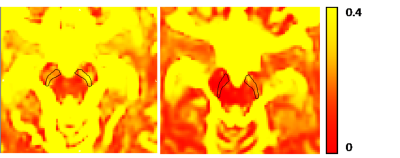
Figure
3: figure shows the freewater maps for (a) sample PD patient and (b)
sample healthy control. It can be clearly observed that the FW levels
in the SNc in PD are high compared to the control. Moreover, in (a)
the left SNc shows higher FW than the right.
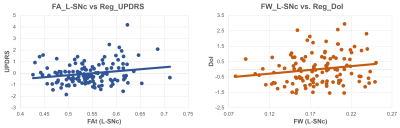
Figure
4: Significant Correlations of (a) FAt
(Left SNc) vs UPDRS scores (b) FW (Left SNc) vs Duration of Illness.