1523
Fibre-specific white matter degeneration in the patients with Progressive supranuclear palsy1Chang Gung University, Taoyuan, Taiwan, 2Chang Gung Memorial Hospital, Linkou, Taoyuan, Taiwan, 3Chang Gung Memorial Hospital, Taoyuan, Taoyuan, Taiwan
Synopsis
Progressive supranuclear palsy (PSP) is an atypical Parkinsonism but with a faster progressive course. Previous studies indicated that PSP patients showed not only gray matter volume decrease but also white matter tract degeneration. We use fixel based analysis to examine the difference in the fibre bundle (FD), fibre-bundle cross-section (FC) and combination of fibre density and bundle cross-sectional area (FDC) between patients with PSP and healthy controls. The results show that significant degeneration of white matter in PSP patients. The major advantage to this study is providing a fixel-based comparison that indicate more directly interpretable measures of structural integrity.
Introduction
Progressive supranuclear palsy (PSP) is an atypical Parkinsonism which shares similar clinical motor symptoms with Parkinson’s disease (PD)1 but with a faster progressive course2. Although studies reported gray matter atrophy in the thalamus, insula, frontal and temporal gyri3,4, PSP can be characterized by the pathological changes and degeneration affecting the white matter, particularly in the cortocispinal tract and cerebellar peduncle5,6.Fixel based analysis (FBA) has been used to characterize multiple fibre orientations within the voxel of interest7. This technique allowed us to measure the total intra-axonal volume of white matter axons, therefore providing a method that can be used to detect degeneration within white matter tracts. FBA can be used to evaluate density of fibres within a fibre bundle (FD), fibre-bundle cross-section (FC) and combination of both fibre density and bundle cross-sectional area (FDC)8.
This study aimed to investigate the pattern of white matter degeneration in patients with PSP. We examined the difference in the FD, FC and FDC between patients with PSP and healthy controls.
Methods
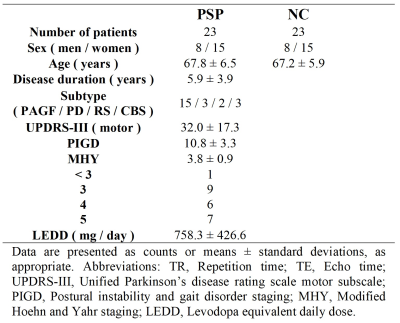
Image AcquisitionImages were acquired from 23 patients (8 men and 15 women; mean age: 67.8 ± 6.5 years) with PSP and 23 age and sex-matched healthy control subjects (mean age: 67.2 ± 5.9 years) using a 3T MR scanner (Magnetom Trio; Siemens, Erlangen, Germany). A summary of clinical and demographic data was listed in Table 1. Multi-shell diffusion-weighted images were acquired with spin-echo echo-planar imaging (SE-EPI) sequence using the following parameters: TR/TE = 5700 ms/108 ms, voxel size = 2 × 2 × 3 mm3. 40 slices were acquired to cover the brain above the cerebellum. Diffusion-weighted gradients were applied along 30 non-collinear directions using b-values of 0, 1000, 2000, and 3000 s/mm2.
A total of 160 contiguous axial T1-weighted images were acquired with magnetization-prepared rapid acquisition gradient-echo sequence (T1-MPRAGE) using the following parameters: TR/TE = 2000 ms/2.63 ms; flip angle = 9°; field of view =224 mm × 256 mm, matrix size = 224 × 256 − resulting in a voxel size of 1 mm × 1 mm × 1 mm.
Fixel Based Analysis
The analysis of fixel followed Raffelt et al.7 Image preprocessing steps including denoising, Gibbs ringing removal, motion and distortion correction, bias field correction were performed in MRtrix37. Each study participant was registered to a study-specific template11 to compute fixel-based derived metrics. Multi-tissue constraint spherical deconvolution (CSD) variants were used for computing fibre density (FD) and fibre cross-section (FC) with fibre orientation distributions (FODs)9. A combined index fiber density and cross-section (FDC) was calculated by multiplying FD and FC7. The white matter regions were parcellated according to ICBM-DTI-81 white matter labels atlas12.
Statistical analysis
Pearson’s chi-square test was used for categorical data. Family-wise error (FWE)-corrected P-values were then assigned to each fixel using non-parametric permutation testing and connectivity-based fixel enhancement (CFE)8. Significant fixels (FWE-corrected P-value < 0.05) were then displayed using the mrview tool in MRtrix3. The changes of fixel-based metrics were subsequently calculated for each significant fiber bundles.
Results
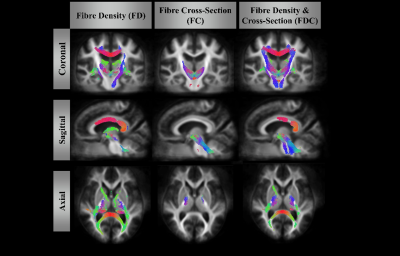
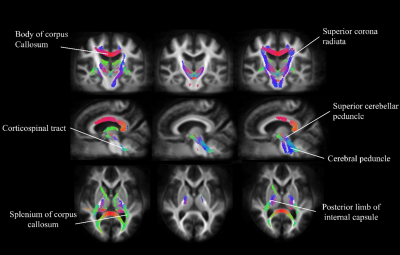
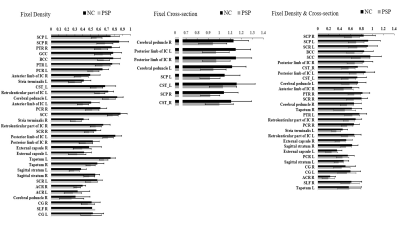
Figure 1 and 2. showed significant reduction in FD (left column), FC (middle column) and FDC (right column) from patients with PSP. FD alteration appeared in the body as well as splenium of corpus callosum and cortical spinal tract. A significant decrease in FC was identified in the bilateral part of the posterior limb of the internal capsule, superior cerebellar peduncle. The distribution patterns are similar between FD and FDC.The difference of the mean values from fixels with significant change in each white matter regions was shown in Figure 3. The regions with FD reduction are mainly located at superior cerebellar peduncle, posterior thalamic radiation, genu and body of corpus callosum, posterior corona radiata, anterior limb of internal capsule, stria terminalis, cortical spinal tract. Significant reduction of FC was found in the posterior and anterior limbs of limb of internal capsule, cortical spinal tract, splenium of corpus callosum, cerebral peduncle, superior longitudinal fasciculus, stria terminalis, cingulate gyrus. Main FDC reduction was found in superior cerebellar peduncle, superior corona radiata, body and genu of corus callosum, posterior limb of internal capsule, cortical spinal tract and cerebral peduncle.
Discussion
Our findings demonstrated a specific degeneration pattern of white matter in patients with PSP. Selective degeneration along specific fibres can be identified by fixel based analysis in patients with PSP; noticeably in the superior cerebral peduncle, corpus callosum and the corticospinal tracts. The locations of fixels with significant changes were congruent with the regions of white matter damage reported by previous DTI literature14,15. Because the changes in FD can be attributed to axonal loss and FC to the morphological alteration in the fiber cross-section, the major advantage to this study is to provide a comparison with more directly interpretable measures of structural integrity. The affected white matter region likely plays an important role in clinical dysfunction for patients with PSP.Acknowledgements
No acknowledgement found.References
1. Kurata T, Kametaka S, Ohta Y, et al. PSP as distinguished from CBD, MSA-P and PD by clinical and imaging differences at an early stage. Intern Med. 2011;50(22):2775-81.
2. Golbe L, Davis P, Schoenberg B, et al. Prevalence and natural history of progressive supranuclear palsy. Neurology. 1988;38(7):1031-1031.
3. Fang Y, Daniel B, Bundhit T, et al. Patterns of gray matter atrophy in atypical parkinsonism syndromes: a VBM meta-analysis. Brain Behav. 2015;5(6):e00329.
4. PingLei P, Yi L, Yang Z, et al. Brain gray matter abnormalities in progressive supranuclear palsy revisited. Oncotarget. 2017;8(46):80941-80955.
5. Jennifer W, Ankit M, Ramesh A, et al. Clinical correlates of white matter tract degeneration in PSP. Arch Neurol. 2011;68(6):753-760.
6. Armstrong R. White matter pathology in progressive supranuclear palsy (PSP): a quantitative study of 8 cases. Clin. Neuropathol. 2013;32(5):399-405.
7. Raffelt D, Tournier D, Smith E, et al. Investigating white matter fibre density and morphology using fixel-based analysis. Neuroimage. 2017;144(Pt A):58-73.
8. Raffelt D, Smith E, Ridgway R, et al. Connectivity-based fixel enhancement: Whole-brain statistical analysis of diffusion MRI measures in the presence of crossing fibres. Neuroimage. 2015;117:40-55.
9. Jeurissen B, Tournier D, Dhollander T, et al. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage. 2014;103:411-426.
10. Raffelt D, Tournier D, Rose S, et al. Apparent Fibre Density: a novel measure for the analysis of diffusion-weighted magnetic resonance images. Neuroimage. 2012;59(4):3976-3994.
11. Tournier D, Calamante F, Connelly A. Determination of the appropriate b value and number of gradient directions for high-angular-resolution diffusion-weighted imaging. NMR Biomed. 2013;26(12):1775-1786.
12. Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40(2):570-582.
13. Mori S, Wakana S. MRI atlas of human white matter. Elsevier. May 11, 2005.
14. Piattella MC, Upadhyay N, Bologna M, et al. Neuroimaging evidence of gray and white matter damage and clinical correlates in progressive supranuclear palsy. J Neurol. 2015;262:1850-1858.
15. Agosta F, Galantucci S, Svetel M, et al. Clinical, cognitive, and behavioural correlates of white matter damage in progressive supranuclear palsy. J Neurol. 2014;261:913-924.
Figures