1510
Quantitative Evaluation of Impaired Neuroenergetics in Parkinson’s Disease and the Treatment Effects of Ursodeoxycholic Acid1CMRR, Department of Radiology, University of Minnesota, Minneapolis, MN, United States, 2Department of Experimental and Clinical Pharmacology, University of Minnesota, Minneapolis, MN, United States, 3Department of Neurology, University of Minnesota, Minneapolis, MN, United States, 4Department of Neurosurgery, University of Minnesota, Minneapolis, MN, United States, 5Departments of Medicine and Genetics, Cell Biology and Development, University of Minnesota, Minneapolis, MN, United States, 6Department of Food Science and Nutrition, University of Minnesota, Minneapolis, MN, United States
Synopsis
Abnormal energy metabolism due to mitochondrial dysfunction is thought to be a major contributor to the progression of Parkinson’s disease (PD). We employed 31P MRS-MT technique at 7T to quantify key bioenergetic parameters in the occipital lobe of people with PD (PWPs). Significantly lower intracellular ATP concentrations together with elevated ATPase activity was found in PWPs; suggesting that augmented ATPase enzymatic activity may represent a compensatory mechanism to bioenergetic deficits that occur in PD. The FDA-approved drug, ursodeoxycholic acid (UDCA), shown to have energy-enhancing properties was evaluated for its effect on improving neuroenergetics in PWPs using the 31P MRS-MT approach.
INTRODUCTION
Parkinson’s disease (PD) is associated with abnormal mitochondrial function and impaired cerebral energy metabolism that evolves over time and plays a critical role in the cause and progression of the disease.1,2 In this study, we applied in vivo 31P MRS in combination with magnetization transfer (31P MRS-MT)3,4 at 7T to measure the concentration and production rate of adenosine triphosphate (ATP) in the occipital lobe of people with Parkinson’s disease (PWPs) where abnormal ATP metabolism has been previously detected.5,6 In addition, we conducted a preliminary study to evaluate the effect of the mitochondrial-enhancing bile acid ursodeoxycholic acid (UDCA)7 on improving brain ATP production in PWPs.METHODS
Study Participants: Eleven patients with mild-moderate Parkinson’s disease (PD, 64±8 years old, 5 males and 6 female, UDPRS scores=36±11) and an equal number of age/gender-matched controls (CT, 61±8 years old, 5M/6F) were recruited for the study. The Montreal Cognitive Assessment (MoCA) scores for PD and CT groups were 28±2 and 29±1, respectively, and were considered normal. University of Minnesota IRB committee approved the study protocol.In vivo 31P MRS-MT measurement and data analysis: The in vivo 31P MRS-MT study was conducted on a 7T/90cm actively shielded human scanner (Siemens MAGNETOM) using a 1H/31P (Dia.≈ 5cm) surface coil probe placed over the occipital lobe. After anatomic imaging and B0 shim, 31P spectra with and without γ-ATP resonance saturation,8 respectively, were acquired using the following parameters: 300µs hard pulse with optimized power and flip angle for excitation, TR=3s and NT=320. For absolute quantification of metabolite concentrations, 3D-CSI (FOV=12×12×9cm3, matrix=7×7×5, TR=1.2s, total NT=896) was acquired on each subject and an ATP phantom.9 AMARES algorithm10 in jMRUI software was used for fitting and analyzing the 31P MR spectra, and metabolite concentrations were quantified using ATP as an internal standard.9 The forward reaction rate constant of ATPase (kf,ATPase) and CK (kf,CK) were determined based on the T1nom method.11 The cerebral metabolic rate of ATP production via ATPase (CMRATP) or CK (CMRCK) reaction was determined by multiplying the kf with [Pi] or [PCr], respectively.
UDCA Therapy: A 6-week study of repeated oral dose of UDCA was conducted in three PD patients. The UDCA dosages were: week 1=15mg/kg/day, week 2=30mg/kg/day and weeks 3-6=50mg/kg/day. The 31P MRS-MT measurements were performed before and after the 6-week UDCA treatment.
RESULTS
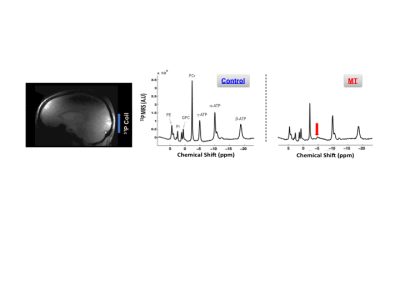
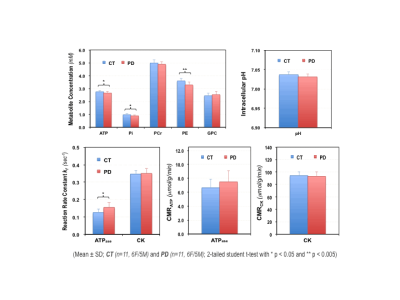
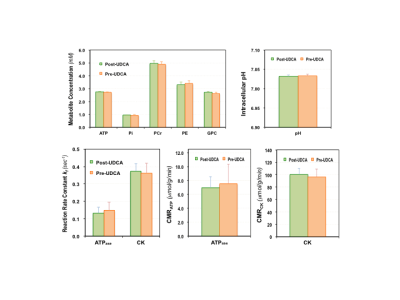
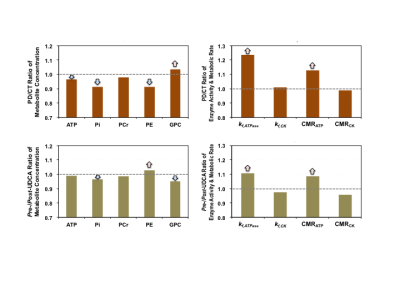
Figure 1 shows typical 7T 31P MRS-MT and 1H MRI data from a representative subject. Figure 2 summarizes the cerebral ATP, PCr, Pi, phosphoethanolamine (PE) and glycerophosphocholine (GPC) concentrations, intracellular pH, forward rate constant and cerebral metabolic rate of ATPase and CK reactions measured in PWPs and control subjects. Significantly lower ATP levels, enhanced ATPase enzyme activity and ATP production were detected in PD brains. Figure 3 displays the same parameters of three PWPs before and after oral UDCA treatment. We found that the [ATP], [Pi], kf,ATPase, and CMRATP in PD shifted toward controls’ values after the 6-week UDCA regimen, while [PE] and [GPC] were further away from that of controls. Such metabolic alterations were clearly demonstrated in Figure 4, where the neuroenergetic changes between the PD and CT groups, or the pre-UDCA and post-UDCA treatment were represented by the ratios of the parameters measured under those two conditions.DISCUSSION and CONCLUSION
Impaired mitochondrial function occurs early in neurodegeneration and ultimately leads to energy failure and cell death.12 Therefore, the ability to directly study ATP energy metabolism in the human brain may provide insights into the pathophysiology of the neurodegenerative diseases such as PD and/or allow quantitative evaluation of treatment efficacy. In this study, we applied 31P MRS-MT technique to directly measure the ATP levels and ATP production rate in the occipital region.5,6 Our results confirm that the [ATP] and [Pi] levels were indeed significantly lower in PD; furthermore, we found that the ATPase enzyme activity (represented by kf,ATPase) and CMRATP were higher in PD than those of controls. We hypothesized that a cellular energy compensation mechanism might be present in PD, during which brain cells attempt to maintain ATP homeostasis by increasing ATPase activity and ATP production. Additionally, we used these energetic parameters to evaluate UDCA, a naturally occurring bile acid and FDA-approved drug for treating primary biliary cholangitis, for its effects on improving mitochondrial function in PD.7 Based on limited data, we determined that orally-administered UDCA seems to improve the [ATP] availability and normalize the ATPase activity and CMRATP; and thus, may have a beneficial disease-modifying effect in PD. Of note, UDCA also affects cell membrane activities, which were altered in the diseased brain. Interestingly, the precursor and intermediate levels of the phosphorus lipid metabolism, i.e., [PE] and [GPC] that are quantifiable via the 31P MRS measurement, showed opposite changes for pre-/post-UDCA vs. the PD/CT conditions, though the interpretation is not clear and requires further investigation.In conclusion, 31P MRS-MT-based neuroimaging is able to directly and non-invasively assess key neuroenergetic parameters, and thus, may be used to better understand PD and possibly evaluate for a response to investigational treatments in those with PD.
Acknowledgements
University of Minnesota Academic Health Center Faculty Research Development Grant; NIH Grants: R01 MH111413, R01 CA240953, R24 MH106049, U01EB026978, P41 EB027061, P30 NS076408, and the University of Minnesota Foundation. Lastly, we thank the individuals with PD and control subjects who volunteered and made this study possible.
References
- 1. Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006;443(7113):787-795.
- 2. Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell 2012;148(6):1145-1159.
- 3. Lei H, Ugurbil K, Chen W. Measurement of unidirectional Pi to ATP flux in human visual cortex at 7 T by using in vivo 31P magnetic resonance spectroscopy. PNAS; 100:14409-14414 (2003).
- 4. Du F, Zhu XH, Qiao H, Zhang X, Chen W. Efficient in vivo 31P magnetization transfer approach for noninvasively determining multiple kinetic parameters and metabolic fluxes of ATP metabolism in the human brain. MRM; 57:103-14 (2007).
- 5. Zhu XH, Lee BY, Rolandelli S, Tuite P and Chen W. Preliminary study of cerebral NAD metabolism and redox state in Parkinson’s patients. Proc ISMRM 22: 480 (2014).
- 6. Zhu XH, Lee BY, Rolandelli S, Tuite P and Chen W. Cerebral phosphorus metabolites profiling of Parkinson’s disease patients at 7T. Proc ISMRM 22: 2395 (2014).
- 7. Vang S, Longley K, Steer CJ, Low WC. The unexpected uses of urso- and tauroursodeoxycholic acid in the treatment of non-liver diseases. Glob Adv Health Med. 2014;3:58-69.
- 8. de Graaf R A, Luo Y, Garwood M and Nicolay K. B1-insensitive single-shot localization and water suppression. J. Magn. Reson. B 1996;113, 35–45.
- 9. Zhu XH, Lee BY and Chen W. Functional energetic responses and individual variance of the human brain revealed by quantitative imaging of adenosine triphosphate production rates. J Cereb Blood Flow Metab. 2018;38(6):959-972.
- 10. Vanhamme L, van den Boogaart A and Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129(1):35-43.
- 11. Xiong et al., ATP production rate via creatine kinase or ATP synthase in vivo: a novel superfast magnetization saturation transfer method. Circ. Res; 108: 653-663 (2011).
- 12. Pathak D, Berthet A, Nakamura K. Energy failure: does it contribute to neurodegeneration? Annals of neurology 2013;74(4):506-516.
Figures