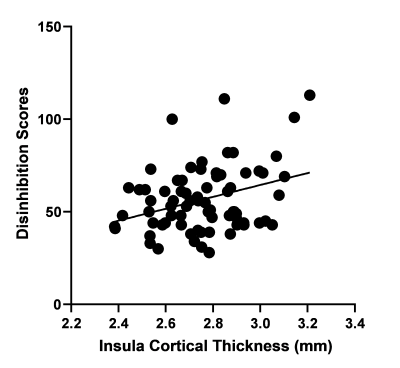1507
Insula structural changes and behavioral disinhibition in Parkinson’s Disease1Neurology, Vanderbilt University, Nashville, TN, United States, 2Psychology, Vanderbilt University, Nashville, TN, United States, 3Biomedical Engineering, Vanderbilt University, Nashville, TN, United States, 4Neuropsychiatry, Vanderbilt University Medical Center, Nashville, TN, United States, 5Psychiatry, Vanderbilt University Medical Center, Nashville, TN, United States, 6Neurology, Vanderbilt University Medical Center, Nashville, TN, United States
Synopsis
Parkinson’s Disease (PD) patients who take dopaminergic therapy are at risk for developing impulsive-compulsive behaviors, and these behaviors localize to the mesocorticolimbic regions. Using a caregiver-reported values from the Frontal Systems Behavioral Scale (FrSBe), we assessed disinhibition, apathy, and dysexecutive symptoms in 72 PD patients. All participants completed brain MRI, and we measured cortical thickness in frontal regions, assessing the relationship between cortical thickness and FrSBE scores. We find that thickness in the insula is directly related to disinhibited behaviors. These results provide new insights into how cortical changes and behavioral symptoms are linked in PD.
Introduction
Dopaminergic medications used in the treatment of the motor symptoms of Parkinson’s Disease (PD) can modulate mesocortical regions, such as the insula, orbitofrontal cortex, and ventromedial prefrontal cortex. An overarching hypothesis that accounts for treatment-related behavioral changes is that dopaminergic therapies can result in an ‘overactivation’ of the reward circuit. This circuit includes the ventral striatum, insula, and orbitofrontal cortex. Functional neuroimaging studies in PD patients with behavioral dysregulation indicate that dopamine therapy can increase ventral striatal metabolism due to altered dopamine regulation, there is increased connectivity between ventral striatal and mesocortical regions. Previous studies rely on the Questionnaire for Impulsive-Compulsive Disorders (QUIP) in Parkinson’s Disease, and, while the QUIP is useful for determining type and severity of a range of behaviors, it lacks some of the broader impulsive cognitive changes and may not account for patient’s own (lack of) insight into their behavioral changes. The Frontal Systems Behavioral Scale (FrSBE), is an established scale designed to measure frontal systems behavioral syndromes commonly affected in Parkinson’s patients: apathy, disinhibition, and executive dysfunction. The purpose of this study is to explore the more reliable caregiver-reported changes in these behavioral domains as they relate to frontal cortical thickness in Parkinson’s patients.Methods
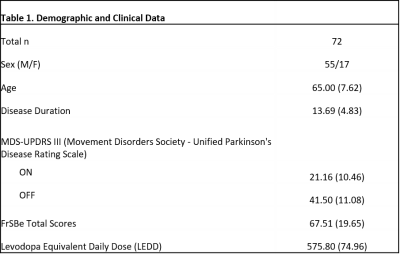
Participants: We examined 72 PD patients who were currently taking dopaminergic therapy. A neurologic exam was performed on all participants, and caregivers completed the FrSBE. Demographic and clinical data are shown in Table 1.Image Acquisition: Patients were scanned in the ON-medication state using a 3T MRI scanner (Achieva, Philips Healthcare, the Netherlands) with body coil transmit and 32-channel head coil reception. Scanning included a 3D structural T1-weighted whole-brain image, (MPRAGE, TR/TE=8.9/4.6 ms; turbo gradient echo factor=131; spatial resolution=1x1x1 mm3).
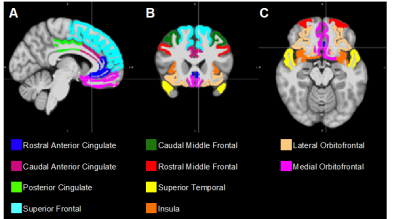
Image Analysis: Regions of interest (ROIs) including the caudal anterior cingulate, caudal middle frontal, lateral orbitofrontal, medial orbitofrontal, posterior cingulate, rostral anterior cingulate, rostral middle frontal, superior frontal, superior temporal and insula were selected due to previous evidence of their importance in frontal-striatal mediated behaviors1–4, and segmented on each subject’s T1-weighted image using FreeSurfer (Version 6.0, http://surfer.nmr.mgh.harvard.edu). Cortical parcellation was performed using the Desikan-Killiany atlas (Figure 1).
Statistics: To evaluate the relationship between behavioral changes and cortical thickness, we used a general linear model using FrSBe scores (total score and each of the 3 sub-scale scores: apathy, disinhibition, and executive dysfunction) as dependent variables, cortical thickness as the independent variable, and age and sex as covariates. These analyses were performed separately for every ROI, and to account for multiple comparisons, the results were considered significant at the level of false discovery rate (FDR) of 0.10.
Results
After correcting for age and sex, we find a significant positive correlation between disinhibition sub-scale score and cortical thickness in the insula (p = 0.005, Figure 2). After applying FDR corrections, there were no significant associations between cortical thickness in other ROIs with either total FrSBE score, apathy sub-scale scores, or executive dysfunction sub-scale scores.Discussion
Insula activation may impact prefrontal cortical functioning, such that it subverts attention, reasoning, and planning abilities and ‘hijacks’ the cognitive resources necessary for exerting inhibitory control5,6. Our results do not replicate previous findings of cortical atrophy in patients with impulsive and compulsive behaviors3, but we applied a caregiver assessment of behavioral changes. Hypertrophy of the insula may be a result of either an increased activation of the reward system or may suggest that patients with increased levels of disinhibition have a more preserved mesocortical region and are more susceptible to dopaminergic effects. Further studies should explore potential differences in other mesocortical areas, including subcortical structures. Additionally, future studies may benefit from employing an atlas that defines regions of the brain in a multimodal manner7 that may provide better neuroanatomical precision into relating structural changes to individual changes.Acknowledgements
This study was supported by the NIH/NINDS R01NS97783 and NIH/NCATS UL1 TR000445. We would like to thank all the patients who volunteered for this study, and a special thank you to Katie Hay and Shelby Hughes for help in the collection and organization of patient data.References
1. Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32(1):56-67. doi:10.1016/j.tins.2008.09.009
2. Dalley JW, Everitt BJ, Robbins TW. Impulsivity, Compulsivity, and Top-Down Cognitive Control. Neuron. 2011;69(4):680-694. doi:10.1016/J.NEURON.2011.01.020
3. Biundo R, Weis L, Facchini S, et al. Patterns of cortical thickness associated with impulse control disorders in Parkinson’s disease. Mov Disord. 2015;30(5):688-695. doi:10.1002/mds.26154
4. Kubera KM, Schmitgen MM, Nagel S, et al. A search for cortical correlates of trait impulsivity in Parkinson´s disease. Behav Brain Res. 2019;369:111911. doi:10.1016/J.BBR.2019.111911
5. Bechara A. Decision making, impulse control and loss of willpower to resist drugs: A neurocognitive perspective. Nat Neurosci. 2005. doi:10.1038/nn1584
6. Contreras M, Ceric F, Torrealba F. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science (80- ). 2007. doi:10.1126/science.1145590
7. Glasser MF, Coalson TS, Robinson EC, et al. A multi-modal parcellation of human cerebral cortex Europe PMC Funders Group. Nature. 2016;536(7615):171-178. doi:10.1038/nature18933
Figures