1500
Quantitative assessment of abnormal susceptibility, R1 and R2* changes in the deep gray matter nuclei in Parkinson’s disease and essential tremor1Radiology, Wayne State University, Detroit, MI, United States, 2Biomedical Engineering, Wayne State University, Detroit, MI, United States, 3SpinTech, Bingham Farms, MI, United States, 4Radiology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, 5Neurology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, 6MR Medical Imaging Innovations, Telangana, India
Synopsis
This work proposes two investigated problems. The first is the separation of confounding tissue properties of deep gray matter using R1, R2*, and QSM from a 3D GRE protocol known as STAGE by sampling a distribution of low and high iron regions across subjects, including very high iron regions in aceruloplasminemia. The second is investigating if we see any differences in these structures in Parkinson’s Disease, essential tremor, and healthy control subjects. These problems are investigated to show that susceptibility changes and R1 are linked, and that water and iron related changes are observable when comparing controls versus Parkinson’s Disease
Introduction
Parkinson’s disease (PD) is a progressive disease with a wide spectrum of motor and non-motor symptoms. It is previously reported that increases in iron content of dopaminergic pathway nuclei, especially in the substantia nigra, and the atrophy of deep gray matter with a resulting increase in water content can be visualized in PD patients along with normal physiological changes due to aging.(1) It is still unclear how the combination of all these confounding factors may contribute to abnormalities seen in PD. STrategically Acquired Gradient Echo (STAGE) imaging is a flow compensated acquisition protocol in which two flip angles and multiple echo times allow for the reconstruction of quantitative MR mapping techniques including the longitudinal relaxation rate (R1), transverse relaxation rate (R2*), and Quantitative Susceptibility Mapping (QSM). In this work, we analyze the deep gray matter (DGM) structures of three groups of subjects; PD and essential tremor (ET) patients along with healthy controls (HC) in order to quantitatively determine whether water and iron content changes in the DGM of PD and ET patients differed from HC in an elderly cohort.Methods
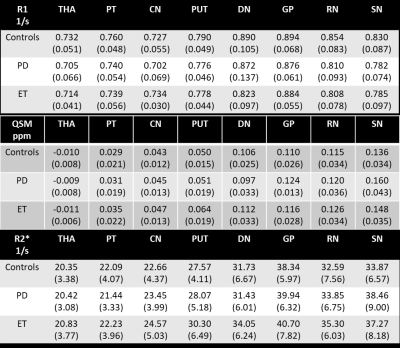
A total of 69 HC subjects (aged 62.9±5 years), 46 PD subjects (aged 63.8±8 years) and 9 ET subjects (aged 64.6±6 years) were scanned on two Philips 3T scanners (Ingenia, Philips Healthcare, Eindhoven, NL) with the same 15-channel head coil. STAGE imaging parameters included: two flip angles (FAs) = 6⁰/24⁰, pixel bandwidth = 220 Hz/pixel, double echo TEs = 7.5/17.5 ms, TR=25ms, parallel imaging factor (iPAT) = 2.4, field of view = 256mm × 192mm, slice thickness = 2mm and an in-plane resolution = 0.67×1.34 mm2. Output maps were generated using STAGE algorithm processing (2-4). In detail, R1 was calculated using a two FA approach with an assumption of white matter T1 = 900ms allowing for a corrective B1-transmit map. R2* was calculated from a T2* linear fit generated from the logarithmic magnitude values across echoes. Finally, QSM was calculated using an iterative approach with BET and phase quality masks (SMART 2.0, The MRI Institute for Biomedical Research, Bingham Farms, MI, USA) using a separate high resolution SWI sequence with the following imaging parameters: FA = 9⁰, pixel bandwidth = 145 Hz/pixel, TE/TR = 11/26 ms, iPAT =2.4, field of view = 256mm × 192mm, slice thickness = 1.34mm and an in-plane resolution = 0.67×0.67 mm2. DGM structures were drawn manually by three experienced raters (ICC for absolute reliability >0.9) and reviewed as a whole for their boundary correctness, as shown in Figure 1. Structures drawn include: Thalamus (THA), Pulvinar Thalamus (PT), Caudate Nucleus (CN), Putamen (PUT), Dentate Nucleus (DN), Globus Pallidus (GP), Red Nucleus (RN), and Substantia Nigra (SN). Intensity values from images were extracted for all regions of interest (ROIs), then compared between groups using two sample two-tailed t-tests (alpha=0.05). Addionally, with the purpose of evaluating possible relationships, average mean R1, R2* and susceptibility values were plotted against each other to assess the correlation in all three cohorts.Results
Mean ± Standard deviations of R1, R2*, and susceptibility values along with the t-test p-values between the cohorts for each parameter are summarized in Table 1 and Table 2, respectively. The PD group exhibited high susceptibility in all DGM structures except the DN while a significant reduction in R1 and a significant increase in R2* were seen only in the SN of PD patients compared with controls. Also, strong linear correlations were found when assessing PD, HC and ET cohorts combined (Figure 2).Discussion and Conclusions
In this work, our main finding was lower R1 in HCs compared to PD patients. Additionally, in accordance with the previous literature, the SN was found to be the only structure with consistently high iron content as seen in R2* and QSM. Another major finding of this work is the strong linear correlations among these parameters, especially R2*-QSM which appears to be very close to what has been reported in the literature. (5) Quantitative MRI allows us to measure well-defined physical parameters such as R1, R2*, and susceptibility, which are not subject to hardware-specific artifacts. (6) Ogg and Steen noted that age-related changes in R1 vary linearly with brain iron concentration and that iron may determine R1 values. (7) We also noted this in comparing R1 with both susceptibility and R2*, two quantitative measures of iron which are highly correlated. It is well-established that the SN shows high iron in R2* and QSM which we also noted. Higher iron content clearly caused an increase in R1.Acknowledgements
No acknowledgement found.References
1. Burciu RG, Ofori E, Archer DB, Wu SS, Pasternak O, McFarland NR, et al. Progression marker of Parkinson's disease: a 4-year multi-site imaging study. Brain. 2017;140(8):2183-92.
2. Chen Y, Liu S, Wang Y, Kang Y, Haacke EM. STrategically Acquired Gradient Echo (STAGE) imaging, part I: Creating enhanced T1 contrast and standardized susceptibility weighted imaging and quantitative susceptibility mapping. Magnetic resonance imaging. 2018;46:130-9.
3. Wang Y, Chen Y, Wu D, Wang Y, Sethi SK, Yang G, et al. STrategically Acquired Gradient Echo (STAGE) imaging, part II: Correcting for RF inhomogeneities in estimating T1 and proton density. Magnetic resonance imaging. 2018;46:140-50.
4. Haacke EM, Chen Y, Utriainen D, Wu B, Wang Y, Xia S, et al. STrategically Acquired Gradient Echo (STAGE) imaging, part III: Technical advances and clinical applications of a rapid multi-contrast multi-parametric brain imaging method. Magnetic resonance imaging. 2019;65:15-26.
5. Ghassaban K, He N, Sethi SK, Huang P, Chen S, Yan F, et al. Regional High Iron in the Substantia Nigra Differentiates Parkinson's Disease Patients From Healthy Controls. Frontiers in aging neuroscience. 2019;11:106.
Figures