1498
Perfusion-Based Biomarkers of Mild Cognitive Impairment in Parkinson’s disease with different MAPT haplotypes using Arterial Spin Labeling MRI1Institute of Biomedical Engineering, Bogazici University, Istanbul, Turkey, 2Behavioral Neurology and Movement Disorders Unit, Department of Neurology, Istanbul Faculty of Medicine, Istanbul University, Istanbul, Turkey, 3Neuroimaging Unit, Hulusi Behcet Life Sciences Research Center, Istanbul University, Istanbul, Turkey, 4Department of Physiology, Faculty of Medicine, Demiroglu Bilim University, Istanbul, Turkey, 5Department of Neuroscience, Aziz Sancar Institute of Experimental Medicine, Istanbul University, Istanbul, Turkey, 6Department of Psychology, Faculty of Arts and Sciences, Isik University, Istanbul, Turkey, 7CorTechs Labs, San Diego, CA, United States, 8Department of Physiology, Istanbul Faculty of Medicine, Istanbul University, Istanbul, Turkey
Synopsis
The main purpose of this study was to define possible brain perfusion deficits in risky gene carriers in Parkinson’s disease (PD) using arterial spin
INTRODUCTION
Microtubule-associated protein tau (MAPT) genotype codes for Tau protein that stabilizes microtubules. MAPT with H1/H1 haplotype has been related with cognitive impairment in Parkinson’s disease (PD).1 It has been reported that abnormal patterns of cerebral blood flow (CBF) could contribute to the diagnosis of cognitive decline in PD. 2-4 Arterial spin labeling MRI (ASL-MRI) measures CBF quantitatively. 5 The aim of this study was to assess the CBF differences between MAPT haplotypes of H1/H1 and H1/H2 in PD by using ASL-MRI at cerebral cortex parcellations obtained from resting-state functional MRI (rs-fMRI).METHODS
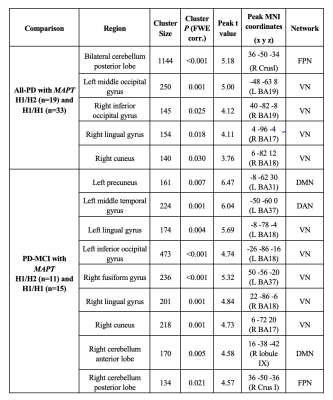
Twenty-seven PD with mild cognitive impairment (PD-MCI), 26 cognitively normal PD (PD-CN), and 15 healthy controls (HC) were included in this study. All participants were scanned on a Philips 3T MR scanner (Philips Medical Systems, Best, The Netherlands) with a 32-channel head coil. ASL-MR images were acquired by signal targeting with alternating radio-frequency labeling using multi-slice single-shot echo-planar imaging readout with Look–Locker sequence at six slices. Single nucleotid polimorphism genotyping for rs4680 (tagging rs9468 MAPT H1 vs. H2 haplotype) was performed on DNA extracted from venous blood samples of all subjects by using Stratagene Mx3005p real-time PCR machine (Agilent Technologies, USA). The acquisition of ASL-MR images was repeated 30 times at each phase to increase signal-to-noise ratio. Also, ASL-MRI data acquisition was conducted three times to cover the whole brain. An in-house software was developed in MATLAB2019a (The MathWorks Inc., Natick, MA) to calculate CBF maps. Then, the CBF maps were registered to MNI152 brain atlas using FMRIB Software Library (FSL). The subgroup of PD patients carrying the risky MAPT H1/H1 haplotype was compared with noncarriers (MAPT H1/H2 haplotype) in terms of CBF by a two-sample t-test. The level of statistical significance was set to uncorrected cluster forming threshold of P < 0.005 and cluster level familywise error (FWE) correction threshold of P < 0.05, with a minimum cluster size of 50 voxels. The MNI coordinates of the statistically significant regions between groups were visually matched to 100 parcellations of the cerebral cortex defined by Schaefer et al. 6 corresponding to Yeo et al.’s 7 seven rs-fMRI networks.RESULTS
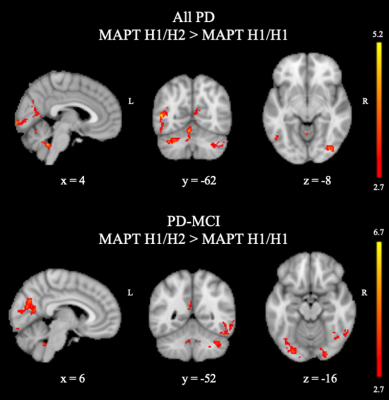
All-PD patients with H1/H1 MAPT haplotype had significantly decreased CBF values compared with those with H1/H2 MAPT haplotype in a large area in the bilateral cerebellum part of fronto-parietal network (FPN) 8; a left middle temporal gyrus (MTG) of visual network (VN), and the anterior hub of FPN (P = 0.001); and three smaller clusters in the right visual network areas, including the inferior occipital gyrus (P = 0.025), lingual gyrus (P = 0.018), and calcarine (P = 0.030) (Table 1). When only the PD-MCI group was considered, PD-MCI patients with MAPT H1/H1 haplotype had lower perfusion in nine areas, six of which were in bilateral visual areas. Two additional areas were cerebellar components of default mode network (DMN) or FPN. Finally, the other region was a posterior medial region comprising the precuneus part of DMN (P = 0.007). The regions of lower CBF in all-PD and PD-MCI patients with the MAPTH1/H1 haplotype are shown in Figure 1.DISCUSSION AND CONCLUSION
The major finding of this study is the posterior hypoperfusion in risky gene carrier PD patients with MAPT H1/H1 haplotype as compared with the ones with MAPT H1/H2 haplotype. MAPT H1 haplotype has been reported to be overrepresented in PD patients. 9 There could be a relationship between visuospatial dysfunction and MAPT H1/H1 haplotype in PD. In conclusion, the assessment of perfusion in PD patients with MAPT H1/H1 haplotype might be used as a biomarker for identifying the disease severity in PD.Acknowledgements
This study was supported by TUBITAK project #115S219 and stanbul University Scientific Research Projects Unit project #1567/42362.The Main Findings.References
1. Nombela C, Rowe JB, Winder-Rhodes SE, et al. Genetic impact on cognition and brain function in newly diagnosed Parkinson's disease: ICICLE-PD study. Brain. 2014; 137: 2743-58.
2. Le Heron CJ, Wright SL, Melzer TR, et al. Comparing cerebral perfusion in Alzheimer's disease and Parkinson's disease dementia: an ASL-MRI study. J Cereb Blood Flow Metab 2014;34(6):964-970.
3. Melzer TR, Watts R, MacAskill MR, et al. Arterial spin labelling reveals an abnormal cerebral perfusion pattern in Parkinson's disease. Brain 2011;134(Pt 3):845-855.
4. Chao LL, Buckley ST, Kornak J, et al. ASL perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheimer Dis Assoc Disord 2010;24(1): 1927.
5. Petcharunpaisan S, Ramalho J and Castillo M. Arterial spin labeling in neuroimaging. World J Radiol. 2010; 2: 384-98.
6. Schaefer A, Kong R, Gordon EM, et al. Local-Global Parcellation of the Human Cerebral Cortex from Intrinsic Functional Connectivity MRI. Cereb Cortex. 2018; 28: 3095-114.
7. Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 2011;106(3):1125-1165.
8. Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 2011;106(5):2322-2345.
9. Winder-Rhodes SE, Hampshire A, Rowe JB, et al. Association between MAPT haplotype and memory function in patients with Parkinson's disease and healthy aging individuals. Neurobiology of Aging. 2015; 36: 1519-28.
Figures