1497
Fixel-based analysis on white matter changes in patients with Parkinson's disease, progressive supranuclear palsy and multiple system atrophy
Nguyen Thanh Thao1, Chih-Chien Tsai2, Yao-Liang Chen3, Jur-Shan Cheng4, Chin-Song Lu5, Yi-Hsin Weng5, Sung-han Lin4, Po-Yuan Chen4, and Jiun-Jie Wang6
1Department of Radiology, Hue University of Medicine and Pharmacy, Hue University, Hue, Vietnam, 2Healthy Aging Research Center, Chang-Gung University, TaoYuan, Taiwan, 3Department of Diagnostic Radiology, Chang Gung Memorial Hospital, Keelung Branch, Keelung, Taiwan, 4Chang-Gung University, TaoYuan, Taiwan, 5Division of Movement Disorders, Department of Neurology, Chang Gung Memorial Hospital, Linkou Branch, TaoYuan, Taiwan, 6Department of Medical Imaging and Radiological Sciences, Chang-Gung University, TaoYuan, Taiwan
1Department of Radiology, Hue University of Medicine and Pharmacy, Hue University, Hue, Vietnam, 2Healthy Aging Research Center, Chang-Gung University, TaoYuan, Taiwan, 3Department of Diagnostic Radiology, Chang Gung Memorial Hospital, Keelung Branch, Keelung, Taiwan, 4Chang-Gung University, TaoYuan, Taiwan, 5Division of Movement Disorders, Department of Neurology, Chang Gung Memorial Hospital, Linkou Branch, TaoYuan, Taiwan, 6Department of Medical Imaging and Radiological Sciences, Chang-Gung University, TaoYuan, Taiwan
Synopsis
White matter degeneration have been attributed to the motor and non-motor symptoms of Parkinson’s disease and atypical parkinsonism. Our study shows different pattern of white matter changes in multiple system atrophy and progressive supranuclear palsy compared to Parkinson’s disease. Furthermore, different affected areas of white matter changes were found among atypical parkinsonism. The involved regions are consistent with the understanding of the pathogenesis of the diseases. Our result proves that fixel based analysis is a robust technique to study white matter degeneration in PD and atypical parkinsonism.
Introduction
Parkinsonism is a neurological disorder characterized by the presence of resting tremor, rigidity, bradykinesia/akinesia and postural instability1. The most common cause of Parkinsonism is the neurodegenerative Parkinson's disease (PD). However, parkinsonism can be caused by various conditions, including at least multiple system atrophy (MSA) and progressive supranuclear palsy (PSP)2. These atypical parkinsonism usually show more rapid functional deterioration than the neurodegenerative PD2. In addition, white matter degeneration might have been involved in the motor and non-motor symptoms of the disorders3–7.Fixel-based analysis is a new approach using higher order diffusion model to compute fiber orientation density function8. It allows the characterization of the micro- and macrostructural environment from single fiber population within each voxel, even with more than one fiber bundles. The aim of this study was to investigate the white matter changes in a cohort of PD, MSA and PSP patients by using fixel-based approach.
Materials and Methods
PatientsThe study has been approved by the institutional review board. 53 patients diagnosed with PD (Male/Female = 29/24, mean age = 65.06±5.51 year old), 47 with MSA (Male/Female = 20/27, mean age = 63.00±7.19 year old) and 50 with PSP (Male/Female = 20/30, mean age = 65.96±3.14 year old) were recruited. The diagnosis was made according to established criteria for PD9, MSA10 and PSP11. All patients were evaluated using Modified Hoehn and Yahr Staging12 and the motor subscale of Unifed Parkinson Disease Rating Scale13.
Data acquisition
Diffusion weighted images were acquired at a 3.0 Tesla scanner (Trio Magnetom; Siemens, Erlangen, Germany) using a 12-channel head coil with a diffusion-sensitive spin-echo EPI sequence. Images of diffusion weighting of b = 1,000 s/mm2 were acquired along 61 non-collinear directions and with a voxel size of 2x2x2 mm3 (89 participants), or alternatively along 30 directions with 2x2x3 mm3 (65 participants).
Image Processing
Fixel-based analysis was performed using MRtrix3 following the procedures by Raffelt et al8,14. Preprocessing steps include Marchenko-Pastur principal component analysis, denoising15, Gibbs ringing removal16, motion and distortion correction17, bias field correction18. Fiber orientations distribution (FOD) in each voxel was estimated using multi-tissue constrained spherical deconvolution19.
A study-specific template was created by spatial normalization in all subjects using symmetric diffeomorphic non-linear transformation FOD-based registration. Fiber density and cross section (FDC) within each voxel was created by multiplying two fixel derived index: Fiber density (FD)8 and fiber bundle cross-section (FC).
Statistical Analysis
Statistical analysis for demographic differences was done by using SPSS (IBM, Armonk, NY, USA). Differences in FDC between groups were calculated using non-parametric permutation testing and connectivity-based fixel enhancement as implemented in MRTrix3, with age, sex, and imaging parameters as covatiates14. A threshold of family-wise error -corrected p < 0.05 and a cluster-extent of 10 or more voxels was considered as statistically significant20.
Results
Figure 1 showed the changes in FDC from PSP when compared to that in patients with PD. In PSP group, reduced FDC was identified in centrum semiovale, corona radiata, commissural fibers of the body of corpus callosum, corticospinal tract, posterior limbs of internal capsule, cerebral peduncles, midbrain and superior cerebellar peduncles. The white matter changes were symmetrical both in the supratentorial and infratentorial compartments. Figure 2 highlighted the changes in bilateral corona radiata (dashed arrows), commissural fibers (arrow head) and superior cerebellar peduncles (black arrows).Figure 3 showed the changes in FDC from MSA group when compared to that in patients with PD. Reduced FDC was noticed in centrum semiovale, corona radiata, corticospinal tract, posterior limbs of internal capsule, cerebral peduncles, midbrain, pons, superior and middle cerebellar peduncles and the arbor viate. It was noticed that the left part of centrum semiovale, corona radiata and middle cerebellar peduncles were more affected than the right side. Figure 4 highlighted the reduction in posterior limbs of internal capsule (black arrows), cerebral peduncles (dash arrows), middle cerebellar peduncles (white arrow head).
The difference of FDC between MSA and PSP was shown in Figure 5. Reduced FDC in patients with PSP was found in bilateral corona radiata and body of the corpus callosum (Upper Row). In contrast, reduced FDC in MSA group was identified in bilateral medial lemniscus and in the left middle cerebellar peduncles (Bottom Row).
Discussions
The study proposed to assess the white matter changes in three different types of patients with parkinsonism by using fixel based analysis. The result showed profound white matter damage in patients with MSA and PSP when compared to that in PD21. The involved regions are consistent with the current understanding of the disease’s pathogenesis. Noticeably, the white matter degeneration was significant in the middle cerebellar peduncles in MSA patients when compared to that in PSP. In contrast, fixel based analysis identified profound alteration in centrum semiovale in PSP patients.Our study showed that fixel-based analysis can provide a comprehensive characterization of the white matter changes in patients with PD, MSA and PSP. It is a robust technique to study which can be sensitive to the underlying changes occurred in the microenvironment of white matter in Parkinson’s disease and in atypical Parkinsonism.
Acknowledgements
No acknowledgement found.References
- McFarland NR. Diagnostic Approach to Atypical Parkinsonian Syndromes. Contin Lifelong Learn Neurol. 2016;22(4 Movement Disorders):1117-1142. doi:10.1212/CON.0000000000000348
- McFarland NR, Hess CW. Recognizing Atypical Parkinsonisms: “Red Flags” and Therapeutic Approaches. Semin Neurol. 2017;37(2):215-227. doi:10.1055/s-0037-1602422
- Bolandzadeh N, Davis JC, Tam R, Handy TC, Liu-Ambrose T. The association between cognitive function and white matter lesion location in older adults: a systematic review. BMC Neurol. 2012;12:126. doi:10.1186/1471-2377-12-126
- Veselý B, Antonini A, Rektor I. The contribution of white matter lesions to Parkinson’s disease motor and gait symptoms: a critical review of the literature. J Neural Transm Vienna Austria 1996. 2016;123(3):241-250. doi:10.1007/s00702-015-1470-9
- Tha KK, Terae S, Yabe I, et al. Microstructural White Matter Abnormalities of Multiple System Atrophy: In Vivo Topographic Illustration by Using Diffusion-Tensor MR Imaging. Radiology. 2010;255(2):563-569. doi:10.1148/radiol.10090988
- Kim H-J, Jeon B, Fung VSC. Role of Magnetic Resonance Imaging in the Diagnosis of Multiple System Atrophy. Mov Disord Clin Pract. 2017;4(1):12-20. doi:10.1002/mdc3.12404
- Whitwell JL, Master AV, Avula R, et al. Clinical correlates of white matter tract degeneration in progressive supranuclear palsy. Arch Neurol. 2011;68(6):753-760. doi:10.1001/archneurol.2011.107
- Raffelt DA, Tournier J-D, Smith RE, et al. Investigating white matter fibre density and morphology using fixel-based analysis. NeuroImage. 2017;144:58-73. doi:10.1016/j.neuroimage.2016.09.029
- Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. Neurology. 1992;42(6):1142-1146. doi:10.1212/wnl.42.6.1142
- Gilman S, Low PA, Quinn N, et al. Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci. 1999;163(1):94-98. doi:10.1016/s0022-510x(98)00304-9
- Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47(1):1-9. doi:10.1212/wnl.47.1.1
- Goetz CG, Poewe W, Rascol O, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord Off J Mov Disord Soc. 2004;19(9):1020-1028. doi:10.1002/mds.20213
- Martínez-Martín P, Gil-Nagel A, Gracia LM, Gómez JB, Martínez-Sarriés J, Bermejo F. Unified Parkinson’s Disease Rating Scale characteristics and structure. The Cooperative Multicentric Group. Mov Disord Off J Mov Disord Soc. 1994;9(1):76-83. doi:10.1002/mds.870090112
- Raffelt DA, Smith RE, Ridgway GR, et al. Connectivity-based fixel enhancement: Whole-brain statistical analysis of diffusion MRI measures in the presence of crossing fibres. NeuroImage. 2015;117:40-55. doi:10.1016/j.neuroimage.2015.05.039
- Veraart J, Novikov DS, Christiaens D, Ades-Aron B, Sijbers J, Fieremans E. Denoising of diffusion MRI using random matrix theory. NeuroImage. 2016;142:394-406. doi:10.1016/j.neuroimage.2016.08.016
- Kellner E, Dhital B, Kiselev VG, Reisert M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn Reson Med. 2016;76(5):1574-1581. doi:10.1002/mrm.26054
- Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage. 2016;125:1063-1078. doi:10.1016/j.neuroimage.2015.10.019
- Tustison NJ, Avants BB, Cook PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29(6):1310-1320. doi:10.1109/TMI.2010.2046908
- Jeurissen B, Tournier J-D, Dhollander T, Connelly A, Sijbers J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. NeuroImage. 2014;103:411-426. doi:10.1016/j.neuroimage.2014.07.061
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15(1):1-25.
- Worker A, Blain C, Jarosz J, et al. Diffusion Tensor Imaging of Parkinson’s Disease, Multiple System Atrophy and Progressive Supranuclear Palsy: A Tract-Based Spatial Statistics Study. PLOS ONE. 2014;9(11):e112638. doi:10.1371/journal.pone.0112638
Figures

Figure 1. FDC changes in PSP compared to that in PD. Reduced FDC in corona
radiata, corpus callosum, internal capsule, cerebral peduncles, midbrain and
superior cerebellar peduncles.
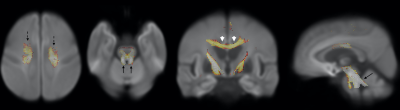
Figure 2. Reduced
FDC in bilateral corona radiata (dashed arrows), commissural fibers of the body
of corpus callosum (arrow head), superior cerebellar peduncles (black arrows) in
PSP group compared to PD group.
Figure
3. FDC changes in MSA compared to that in PD.
Reduced FDC in left corona radiata, internal capsule, cerebral peduncles and
middle cerebellar peduncles.
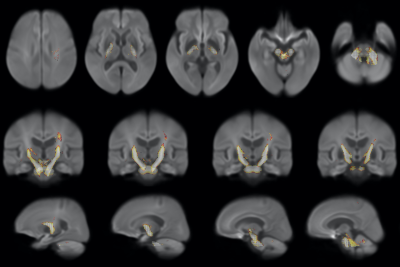
Figure
4. Reduced FDC in posterior limbs of internal capsule (black arrows),
cerebral peduncles (dash arrows), middle cerebellar peduncles (white arrow
head) in MSA group compared to PD group.

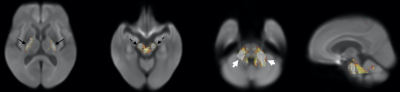
Figure 5.
Reduced FDC in bilateral corona radiata and corpus callosum in PSP compared to MSA
group (upper row). Reduced FDC in bilateral medial lemniscus and in middle
cerebellar peduncles in MSA group compared to PSP (bottom row).