1494
Quantitative susceptibility mapping of brain iron deposition in patients with cerebral small-vessel disease with cerebral microbleeds1Weill Cornell Medicine, Cornell University, New York, NY, United States, 2Shandong Medical Imaging Research Institute Affiliated to Shandong University, Jinan, China
Synopsis
Quantitative susceptibility mapping (QSM) was used to evaluate and compare the characteristics of iron deposition in gray matter nuclei of the brain between patients with cerebral small-vessel disease and cerebral microbleeds (CSVD-CMBs) and those with CSVD and no CMBs(CSVD–no CMBs). The susceptibility values of the bilateral caudate nucleus, putamen, red nucleus, and substantia nigra were significantly higher in patients with CSVD-CMBs than in those with CSVD–no CMBs and in healthy controls. The change in the susceptibility values of these regions may be an imaging marker of cognitive decline in patients with CSVD.
Introduction
Cerebral microbleeds (CMBs) adversely affect the cognitive function of patients with cerebral small-vessel disease (CSVD) and are independent risk factors for cognitive decline (1, 2). Previous studies have shown that in patients with CVSD who have CMBs (CVSD–CMBs), the overall cognitive function decreases (3-6). Brain iron deposition, occurring during neural degenerative changes, may play an important role in mild cognitive impairment (7). Quantitative susceptibility mapping (QSM) is a noninvasive quantitative analysis that was performed in this study to assess brain iron deposition in patients with CSVD–CMBs.Purpose
To explore the correlation between CSVD-CMBs characteristics and cognitive impairment, QSM was used to evaluate and compare the characteristics of iron deposition in the gray matter nuclei of the brain in patients with CSVD–CMBs and in those with CSVD and no CMBs (CSVD–no CMBs).Methods
Patients with CSVD–CMBs, those with CSVD–no CMBs, and healthy control volunteers were included in this study. All participant were right-handed and underwent testing with the Montreal Cognitive Assessment (MoCA). Each participant’s data were collected on a MAGNETOM Skyra 3T MR scanner (Siemens Healthcare, Erlangen, Germany) with a 32-channel head coil. A three-dimensional (3D) fast low-angle shot sequence was run with 10 echoes to obtain magnitude and phase images to evaluate susceptibility values in the brain. 3D T1-weighted structural images were acquired by magnetization-prepared rapid acquisition with a gradient-echo sequence. In addition, T2-weighted turbo spin-echo, fluid-attenuated inversion recovery and diffusion-weighted images were acquired to confirm or rule out any brain abnormalities. The preprocessing of QSM original images conformed to the standard preprocessing steps. With the preprocessed data, the susceptibility value of the region of interest (ROI) was measured by ITK-SNAP (Version 3.8.0) software to reflect the iron content. The ROI included the hippocampus, amygdala, thalamus, nucleus accumbens, caudate nucleus, putamen, globus pallidums, red nucleus, and substantia nigra. Data from the left and right sides were recorded separately. If CMBs were present in the ROI, the corresponding values were excluded to ensure the accuracy of measurement. A senior neuroradiologist performed the assessment of brain disease and pinpointed the ROI. Independent sample t-tests were used to compare the mean MoCA scores and the susceptibility values of the different patient groups and the healthy control volunteers. Spearman correlation analysis was used to determine the correlation between susceptibility values and MoCA scores.Results
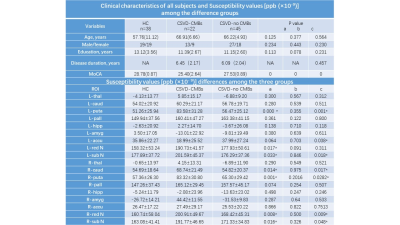
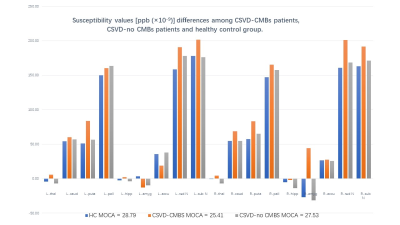
Twenty-three patients with CSVD–CMBs, 46 with CSVD–no CMBs, and 39 healthy control volunteers were included in this study, the details as shown in Figure 1. MoCA scores of the two groups of patients and the healthy volunteers were significantly different (P < 0.05), and patients with CSVD–CMBs had the lowest MoCA scores (25.40 ± 2.64; normal standard, ≥27). The comparison of susceptibility values of the two groups of patients and the healthy volunteers is shown in Figure 1 and Figure 2. We found that the susceptibility values of the left and right caudate nucleus, putamen, red nucleus, and substantia nigra were significantly higher in patients with CSVD–CMBs than in patients with CSVD–no CMBs and in healthy controls (P < 0.05; Figures 3 and 4 ); however, the difference between patients with CSVD–no CMBs and healthy control group in these brain regions was not significant (P > 0.05), and susceptibility values of patients with CSVD–no CMBs tended to be higher in most brain regions than those in the healthy control group (Figure 1). For the patients with CVSD and the healthy volunteers, susceptibility values in multiple brain regions were highly correlated with MoCA scores, as shown in Figure 5.Discussion
The analyses in this study revealed that in comparison with patients with CSVD–no CMBs, those with CSVD–CMBs had more significant abnormal iron deposition in parts of the gray matter nuclei of the brain (mainly the bilateral lobe of caudate nucleus, putamen, red nucleus, and substantia nigra). These regions contain important structures closely involved in cognitive, emotional, and motor functions. CMBs were independent predictors of executive function impairment (8). Previous studies have shown that CVSD accompanied by CMBs can cause a decline in the overall cognitive function, especially in the cognitive areas of visuospatial/executive function, attention, and delayed recall(3). Moreover, patients with Alzheimer’s disease disease who had multiple CMBs had lower scores on the Mini-Mental State Examination(9). Our results also confirmed that patients with CSVD–CMBs showed a significant decline in the cognitive function, and the degree of cerebral iron deposition in these patients was significantly higher than that of patients with CSVD–no CMBs and of healthy control volunteers. The process of iron deposition in the brain during normal aging and neurodegenerative changes may cause neuron damage through oxidative stress (10, 11). The susceptibility values were significantly negatively correlated with MoCA scores, which suggests that cerebral iron deposition exacerbates the decline in cognitive function of patients with CSVD and in the healthy population.Conclusion
Patients with CSVD–CMBs exhibited abnormal iron deposition in the caudate nucleus, putamen, red nucleus, and substantia nigra, and the change in susceptibility values in these regions is likely to be an imaging marker of cognitive decline in patients with CSVD–CMBs.Acknowledgements
This work was supported by a grant from Jinan Scientific and Technological Development Program (CN) (201301049, 201602206, 201907052), Medical and health science and technology development project of Shandong province (2016WS0529), Funding for abroad program by the government of Shandong Province (201803059).References
1. Seo SW, Lee BH, Kim EJ, Chin J, Cho YS, Yoon U, et al. Clinical significance of microbleeds in subcortical vascular dementia. Stroke. 2007;38(6):1949-51.
2. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurology. 2013;12(8):822-38.
3. Uchida Y, Kan H, Sakurai K, Arai N, Kato D, Kawashima S, et al. Voxel-based quantitative susceptibility mapping in Parkinson's disease with mild cognitive impairment. Movement Disorders. 2019;34(8):1164-73.
4. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurology. 2010;9(7):689-701.
5. Haacke EM, Chengb NYC, House MJ, Liu Q, Neelavalli J, Ogg RJ, et al. Imaging iron stores in the brain using magnetic resonance imaging. Magnetic Resonance Imaging. 2005;23(1):1-25.
6. Bozin I, Ge YL, Kuchling J, Dusek P, Chawla S, Harms L, et al. Magnetic Resonance Phase Alterations in Multiple Sclerosis Patients with Short and Long Disease Duration. Plos One. 2015;10(7).
7. Andersen HH, Johnsen KB, Moos T. Iron deposits in the chronically inflamed central nervous system and contributes to neurodegeneration. Cellular and Molecular Life Sciences. 2014;71(9):1607-22.
8. Werring DJ, Frazer DW, Coward LJ, Losseff NA, Watt H, Cipolotti L, et al. Cognitive dysfunction in patients with cerebral microbleeds on T2*-weighted gradient-echo MRI. Brain. 2004;127(Pt 10):2265.
9. Poels MM, Ikram MA, Van ADL, Hofman A, Niessen WJ, Krestin GP, et al. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology. 2011;7(4):326.
10. Carocci A, Catalano A, Sinicropi MS, Genchi G. Oxidative stress and neurodegeneration: the involvement of iron. Biometals. 2018;31(5):715-35.
11. Yang QF, Zhou LN, Liu C, Liu DH, Zhang Y, Li C, et al. Brain iron deposition in type 2 diabetes mellitus with and without mild cognitive impairmentan in vivo susceptibility mapping study. Brain Imaging and Behavior. 2018;12(5):1479-87.
Figures
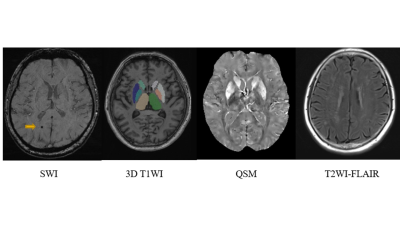
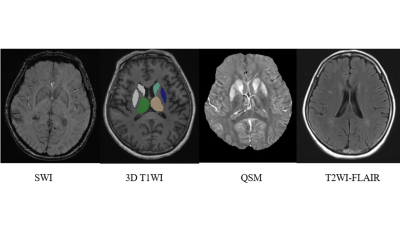
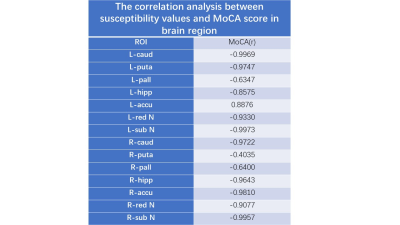
Fugure 5 The correlation analysis between susceptibility values and MoCA score in brain region.
r, correlation coefficient.